| | | | | | | Presented By PhRMA | | | | Axios Vitals | | By Tina Reed · Nov 30, 2022 | | 🐪 Happy Wednesday, Vitals readers. Today's newsletter is 1,091 words or a 4.5-minute read. | | | | | | 1 big thing: New Alzheimer's drug faces uncertain regulatory path |  | | | Illustration: Aïda Amer/Axios | | | | Researchers have at last found a drug that can slow the progression of Alzheimer's disease, according to clinical trial data presented last night. But regulators now have to weigh its relatively modest efficacy against safety risks, Axios' Caitlin Owens and Oriana Gonzalez report. What we're watching: The FDA will soon decide whether to approve Eisai's experimental drug lecanemab — and, if so, for the general public or only certain patient populations. - Medicare will then have to decide whether to pay for it outside of clinical trials or to maintain restrictions it put in place when it considered a similar drug last year.
Driving the news: The results published last night in the New England Journal of Medicine generally matched what the company previewed via press release in September. - The drug slowed cognitive decline in patients in the early stages of the disease by 27% over 18 months.
- "These peer-reviewed, published results show lecanemab will provide patients more time to participate in daily life and live independently. It could mean many months more of recognizing their spouse, children and grandchildren," the Alzheimer's Association said in a statement.
Yes, but: That decline is modest, and some doctors question how meaningful it would be for patients, per the New York Times. What's more, there are some safety concerns about the drug, particularly for patients taking blood thinners. - Two deaths connected to the trial that were recently reported by Science and STAT occurred after the 18-month randomized study period, per the NYT. Both patients received lecanemab in an extension study after the 18 months ended.
What's next: Eisai has submitted an application with the FDA for accelerated approval, and a decision is expected by early January. The company said in September it plans to file for full approval by the end of March. - But the drug's safety concerns are likely to prompt lively debate on whether the agency should limit who can receive it, or at least impose some precautions.
Go deeper. |     | | | | | | 2. To tweet or not to tweet | 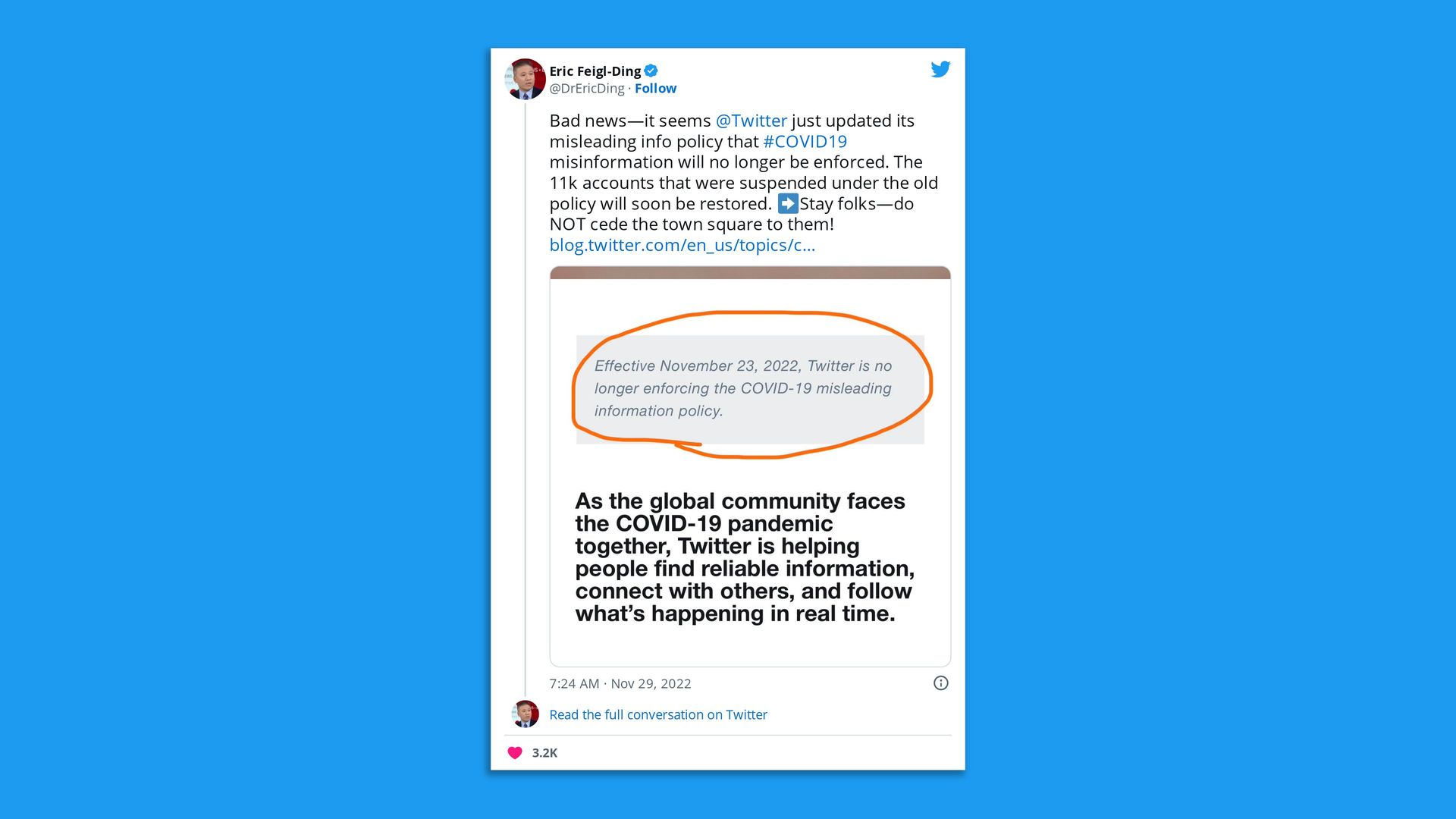 | | | Twitter screenshot. @DrEricDing | | | | Health Care Twitter is still processing the news that the social media platform will no longer enforce its COVID-19 misinformation policy. State of play: Users noticed a post this week on Twitter that read "Effective November 23, 2022, Twitter is no longer enforcing the COVID-19 misleading information policy," Axios' Ivana Saric writes. Of note: Facebook and Instagram's COVID misinformation policies remain in place, a Meta spokesperson told Axios' Sara Fischer. The company has asked its independent Oversight Board to review them. Will they stay or go? "Bad news — it seems @Twitter just updated its misleading info policy that #COVID19 misinformation will no longer be enforced ... Stay folks — do NOT cede the town square to them!" tweeted epidemiologist and health economist Eric Fiegl-Ding. Yes, but: Others, like former CMS acting administrator and Biden health advisor Andy Slavitt, said they're eyeing a move to other platforms. - "I'm also on Mastadon to check it out & until Post is done with its waitlist and will eventually pick one," Slavitt tweeted. "I continue to occasionally check the news feed here & promote things on Twitter minimally & will cross-post for a short time as people decide what they want to do."
Yes, but: Several Vitals reader emails suggest you haven't been riveted to social for your health news so won't be making any changes, regardless of what Musk does. |     | | | | | | 3. Promise for universal flu vaccine | | We may be a step closer to a universal vaccine after an mRNA shot aimed at protecting against 20 different flu strains showed promise in animal tests, the New York Times reports. Why it matters: Universal vaccines are considered a holy grail in protecting against future pandemics, not to mention the annual financial and human costs of fighting the flu. "If there's a new influenza pandemic tomorrow, if we had a vaccine like this that was widely employed before that pandemic, we might not have to shut everything down," Scott Hensley, an immunologist at the University of Pennsylvania who led the work, told the Times. Yes, but: There are a number of challenges for this or any universal vaccine candidate. Among them: simply proving their effectiveness in humans. - "How do you evaluate and regulate a vaccine where their targets aren't circulating, and so you can't really show effectiveness?" Alyson Kelvin, a vaccinologist at the University of Saskatchewan in Canada, told the Times.
The bottom line: There's still a long way to go before this is even tested in humans. But it's a promising reminder of how the pandemic accelerated vaccine science. |     | | | | | | A message from PhRMA | | Data show PBMs shift costs to patients | | |  | | | | Costly out-of-pocket expenses tied to deductible and coinsurance requirements are a leading concern for patients with commercial insurance. New IQVIA data break down how insurers and their PBMs are impacting how patients access and afford their medicines. | | | | | | 4. Data du jour: Deaths from accidental falls rise |  Reproduced from NCHS; Map: Axios Visuals The death rate from accidental falls is rising among seniors, according to data from the CDC's National Center for Health Statistics. Why it matters: It's a leading cause of deaths from unintentional injury among adults aged 65 and over — and expected to continue rising as the population ages. - It's also a costly problem. Every year, about $50 billion is spent on medical costs related to non-fatal fall injuries, including $29 billion paid by Medicare. More than $750 million in spending is connected to fatal falls, per the CDC.
What's happening: Researchers point to a number of contributing factors such as difficulties seeing, walking and balance issues, as well as disabilities, medication effects and walking obstacles, among other factors. By the numbers: Among adults aged 65 to 74, the rate of deaths from unintentional falls for men increased from 11.9 per 100,000 people in 2000 to 23.8 in 2020. For women, they increased from 6.6 to 13.3. - Among adults aged 75 to 85, the rate for men increased from 39.9 in 2000 to 81.6 in 2020.
- For men 85 and up, the rate jumped from 145.4 in 2000 to 329.6 in 2020. It nearly tripled among women from 99.2 in 2000 to 269.8 in 2020.
Our thought bubble: Rates were highest in snowy states like Wisconsin, Vermont, Minnesota, South Dakota and Maine and lowest in southern states like Alabama, Arkansas, Louisiana and Kentucky. |     | | | | | | 5. Catch up quick |  | | | A shipment of winter aid to Ukraine including heated tents, heating stoves and modules for mobile hospitals was prepared Tuesday in Schoenfeld, Germany. Photo: Carsten Koall/Getty Images | | | | 👀 Devastating Russian strikes in Ukraine have cut off power to many hospitals and prompted new warnings about the coming winter. (ABC News) 🩼 That knee surgery you postponed could soon hobble insurance giants (WSJ) 🤒 The flu season has intensified with 6 million infected in the U.S., the CDC says. (The Hill) |     | | | | | | A message from PhRMA | | Data shows insurers and their PBMs shift costs to patients | | |  | | | | Costly OOP expenses tied to deductible and coinsurance requirements are a leading concern for patients with commercial insurance. These harmful practices put in place by insurers and pharmacy benefit managers (PBMs) are even causing patients to abandon their medicines. Learn more. | | | | 👋 Thanks for reading, and thanks to senior editor Adriel Bettelheim and copy editor Nick Aspinwall for the edits. Did someone forward this email to you? Here's how to sign up. |  | | Your personal policy analyst is here. | | | | | | Axios thanks our partners for supporting our newsletters. If you're interested in advertising, learn more here.
Sponsorship has no influence on editorial content. Axios, 3100 Clarendon Blvd, Arlington VA 22201 | | | You received this email because you signed up for newsletters from Axios.
Change your preferences or unsubscribe here. | | | Was this email forwarded to you?
Sign up now to get Axios in your inbox. | | | | Follow Axios on social media:    | | | | | |
No comments