DIgital Health
Charting the wide world of digital health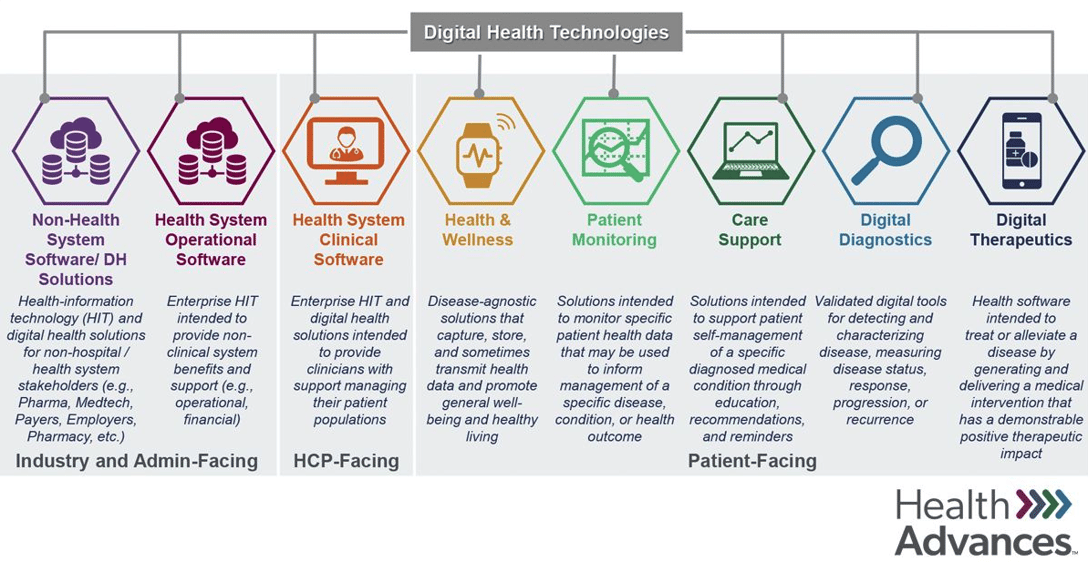
Digital health is an incredibly broad term that can refer to a range of technologies touching very different parts of health care. The chart above, created by consultancy Health Advances, breaks the world of digital health into a series of discrete categories.
The chart also has an agenda: It's from a report sponsored by the Digital Therapeutics Alliance, and digital therapeutics, of course, gets its own category. Still, the chart does an admirable job of capturing the range of efforts in a way that's easier to process than some of the sprawling industry maps you'll occasionally see circulating. What do you all think? Email me!
Research
New study to test targeted approach to Apple Watch A-fib screening
The Apple Watch enables users to monitor themselves for signs of atrial fibrillation, which is no doubt catching asymptomatic cases that were undiagnosed. That's good because catching A-fib allows doctors to take steps to prevent strokes. Still, Apple's technology also carries the risk of false positives, which some speculate in a population of millions of mostly healthy users may result in lots of people seeking expensive care they don't need.
A forthcoming study by researchers at Mayo Clinic aims to see if narrowing the population screened by the Apple Watch to focus on the most vulnerable could offer a path forward. The new work builds on previous studies. In a 2019 paper, a team led by some of the same researchers showed that artificial intelligence could identify people at high risk for A-fib from apparently normal electrocardiograms. A follow-up published last year showed that people deemed high risk by the AI were much more likely to screen positive for A-fib when subjected to continuous monitoring for 30 days.
The new study, set for completion in 2026, will explore if monitoring with an Apple Watch, rather than usual clinical-grade continuous heart monitors, can enable earlier detection of A-fib. Researchers will enroll 2,000 people flagged as high risk by AI and randomize them to receive either an Apple Watch or care as usual. They'll be monitored for a year, which would be very hard to do with a clumsy device with leads attached to the wearer's chest.
"We know that we can risk-stratify a population in terms of atrial fibrillation risk, but what we don't know is what is the most effective way to identify those patients who have atrial fibrillation and get them the treatment they might need," said Peter Noseworthy, a cardiac electrophysiologist at Mayo Clinic and the study's co-principal investor. He added: "We'd like to see if we can move A-fib screening out of the realm of medical care and into the realm of wellness and consumer activity."
Medical Devices
FDA clears Abbott's second leadless pacemaker
Abbott's wireless pacemaker system, which is built to treat patients with slow or irregular heart rhythms, was approved by the Food and Drug Administration on Wednesday. The "dual chamber" system contains two triple A battery-sized pacemakers implanted directly in the right atrium and right ventricle of the heart. Traditional pacemakers sit under the skin and are connected to the heart by leads that can sometimes cause complications and infections.
Vish Charan, Abbott's head of research and development in cardiac rhythm, told STAT's Lizzy Lawrence that while physicians are still getting used to the leadless technology, the company "really believe[s] the future is going to be leadless."
Abbott earned approval for its single chamber system in 2022, and the device maker is betting that its new, two-chamber system will enable it to capture a large share of the market. Charan said 80% of pacemaker patients require the double pacemakers. Medtronic, meanwhile, has been selling its own leadless pacemaker since 2016. Boston Scientific is working on developing a similar device as well.







No comments