Digital health
What's going on at Biofourmis?
Once valued at $1.3 billion, Biofourmis appears to be in a bit of trouble. Yesterday, I reported that the company's founder and CEO Kuldeep Singh Rajput quietly stepped down from his role without having a successor in place, just a month after his company laid off 120 people. Ben Wanamaker — formerly of Humana, Aetna, and Walmart has joined the Biofourmis board — and will oversee the company's leadership until a new chief executive is found.
Over the years, Biofourmis has raised close to half a billion dollars and dabbled in several flavors of tech-enabled care delivery for health systems and digital support for biopharma companies. This summer, the company issued a press release bragging about all its progress over the last year, at the very same time that the layoffs were happening. Now the CEO is out — what's going on?
Read more here.
Regulation
Lawmakers call on FDA to issue stricter bone graft guidance
A bipartisan group of Michigan lawmakers sent the FDA a letter yesterday calling for stronger guidance in testing bone tissue products and screening donors. The statement comes as the CDC works to control a U.S. tuberculosis outbreak that spread via infected bone material used by dentists and orthopedic surgeons. There have been two deaths and at least 36 exposures. It's the second tuberculosis outbreak linked to the biomaterial device company, Aziyo Biologics.
There's no commercially available tuberculosis test for bone tissue. Even if that test existed, neither the FDA nor the American Association of Tissue Banks require companies to test their donor materials for TB.
Sens. Gary Peters and Debbie Stabenow, along with Reps. John Moolenaar and Debbie Dingell, told the FDA they had been contacted by a physician at the Michigan Medicine hospital system whose patient died of TB in August after being implanted with the material. The lawmakers urged the FDA to adopt the American Association of Tissue Banks' recent, more stringent standards for donor screening.
"We urge the FDA to consider these recommendations and promptly
issue guidance or regulations based on sound science to protect patients and increase accountability for human tissue transplant products," they wrote.
Artificial intelligence
Maybe don't ask Alexa for help with CPR
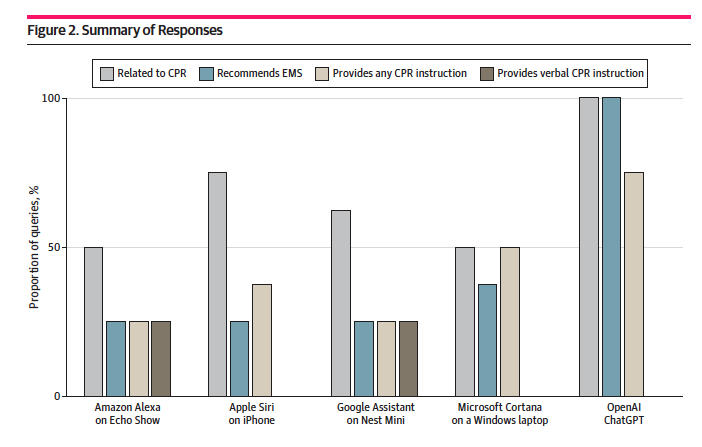
If your dinner companion chokes and is in sudden need of CPR, do not ask Siri or any other voice assistant for help. Your phone is your best friend: Use it to call 911.
That's the takeaway from a new study in which researchers asked four commercial voice assistants as well as OpenAI's ChatGPT a series of different questions about CPR. According to the researchers, the assistants performed poorly, answering with responses about CPR 59% of the time. In several cases, assistants simply said they didn't know. (Microsoft's Cortana twice answered "words fail me" — yikes.) ChatGPT, unsurprisingly, performed much better, but it is unable to speak its responses.
The researchers suggest that voice assistants ought to be programmed to offer CPR instructions using designated CPR commands. Sure! I bet the developers at tech giants are working overtime to get the feature out in time for the holiday shopping season.


No comments