watch this space
A long-awaited era in Apple's health history is here
The Apple Watch is now being leveraged to help manage Parkinson's disease, a degenerative brain disorder that affects as many as a million Americans. Over the past year, the Food and Drug Administration has cleared three Apple Watch apps from developers to track the tremors and dyskinesia associated with Parkinson's — work that began nearly a decade ago.
The goal is to help inform treatment decisions for people and providers, but there's a long slog ahead before doctors and the broader health system can be convinced the tools are worth using. Read Mario's special report on the consumer tech giant's slow inroads in digital health through the lens of a unique disorder.
Budget lines
Time to get picky about health tech investments
After a period of intense optimism and investment — which has left some companies hanging on for dear life after going public at massive overvaluations — investors are course-correcting. Health tech investment dropped by nearly half last year from close to $30 billion in 2021, and the contraction is continuing, with $8.6 billion flowing into health tech startups so far this year.
"There's a high bar in terms of metrics for funding," said Sofia Guerra, a vice president at Bessemer Venture Partners, which recently published its first analysis of the health tech industry. Read more analysis from Guerra and a crop of VCs who spoke with Mohana about responsible growth and looming consolidation.
artificial intelligence
A surge in market-authorized AI devices
The FDA updated its list of artificial intelligence and machine learning-enabled medical tools on Thursday, giving insight into the growth of a device class that remains poorly regulated. The list was first published in 2021, after a STAT investigation found that the agency had failed to keep the public informed on its regulation of AI devices. The FDA's pace of authorizations slowed during the pandemic, but it's revved up this year with 108 added since January. At that rate, 2023 will see authorizations increase more than 30% over last year.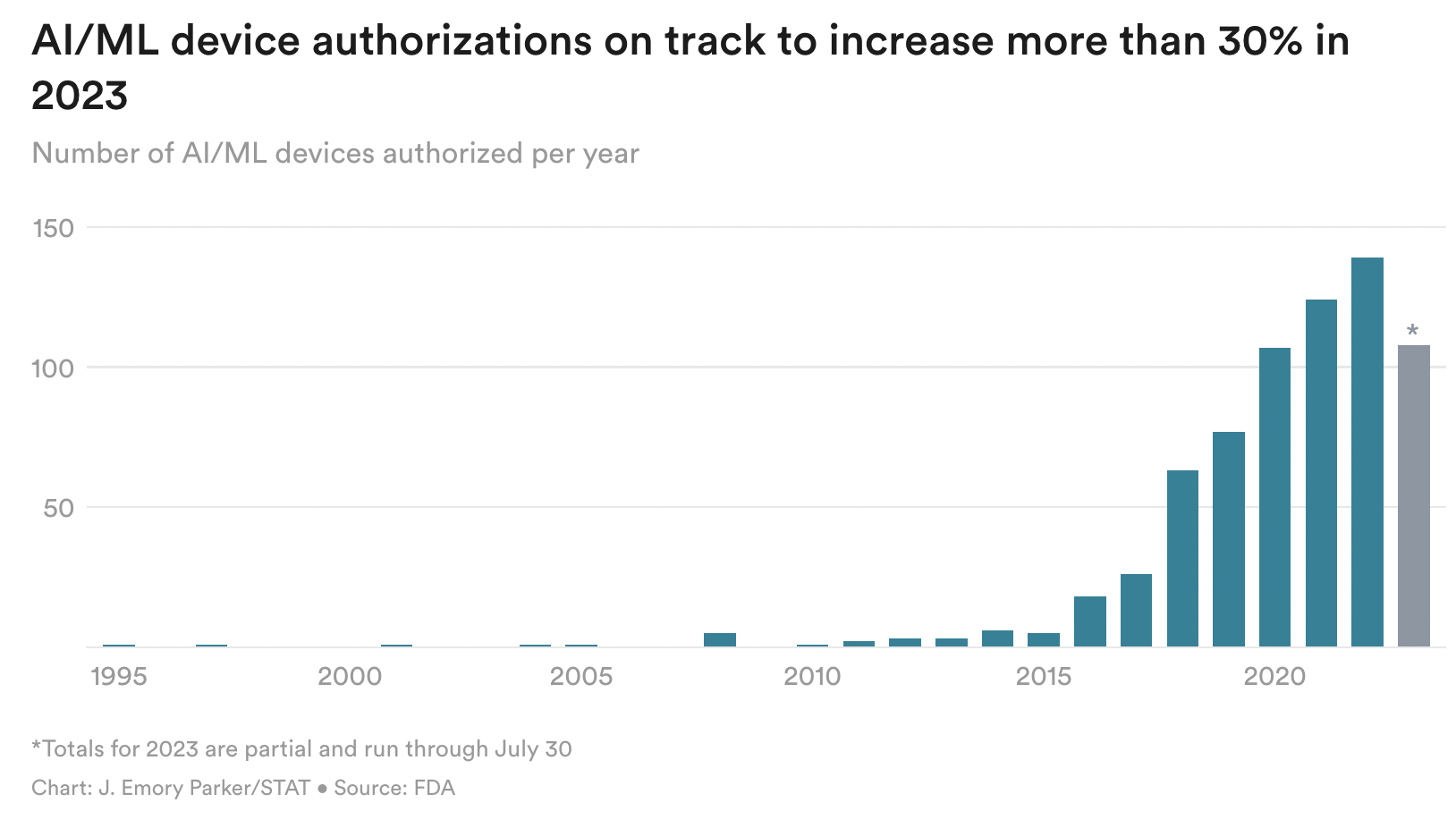
general intelligence
In other FDA news...
- On Monday, the FDA made a major update to its public-facing database for medical device adverse event reports. MAUDE and its API will now include patient age, weight, ethnicity, and race, along with the existing fields used to report device-related injuries, malfunctions, and deaths.
- The agency issued a warning for patients and providers about the risks of compounded ketamine. STAT has previously reported on the risks of at-home use of the drug, which is increasingly marketed by telehealth companies for off-label treatment of mental health disorders.
demos
Doctor vs. ChatGPT
Generative AI is far from ready for primetime in medicine. But with hospitals already putting the technology to the test in their clinicopathological conferences, we decided to run our own (very unscientific) experiment at the STAT Summit in Boston last week. Ann Woolley, an infectious disease specialist from Brigham and Women's Hospital, worked to diagnose two different patient cases — including written histories and medical images — while ChatGPT churned out its answers on a screen beside her.
We can easily declare Woolley the victor in this match-up, though the bot performed surprisingly well on a complex case of a secondary infection in a patient being treated for multiple myeloma. Read more to find where it stumbled, or watch the whole demo on STAT's YouTube channel.
No comments