Alphabet With wastewater deal, Verily scores its first CDC contract

Since its 2015 launch, the Google X moonshot life sciences spinout Verily has dabbled in a wide range of disparate health-related projects, loosely joined by the company's mission to apply sophisticated data analytics to problems entrenched in hospital operations, clinical care, and even drug trials. Over the past few years, its offering have spanned tech-based diabetes management, a brick-and-mortar substance use rehabilitation clinic in Ohio, to facilitating clinical trials for major depressive disorder. Unfortunately, the company's never been able to spin these projects into a viable business, and it's still reporting losses each year.
Its latest contract — a $38 million, up to five-year deal with the Centers for Disease Control and Prevention to handle its wastewater surveillance operations — could portend the beginning of a longer relationship with the federal government and with public health departments. Or it could be just the latest in a series of health-themed projects that have yet to produce a profit.
Verily's leaders told me it's a win for the company and its nascent wastewater division, which launched in 2020 shortly after CDC began tracking wastewater pathogens in earnest. Since then, Verily's worked with a handful of academic centers and some state and local public health departments to use tech like robot-assisted liquid handling and digital droplet analyses.
The wastewater contract could be a "really important cornerstone to demonstrate the value this could have in a large scale population," Andrew Trister, the former Apple Health executive who now serves as Verily's chief scientific officer, told me. Read more.
Medical devices
How to protect patients from risky devices
Lizzy Lawrence sat down with Madris Kinard, a former Food and Drug Administration official who launched Device Events, an online hub gathering all medical device adverse event reports filed to the FDA . The site is frequented by clients like hospitals and law firms but also activists and journalists. She even shared some of those reports with with Erin Brokovich, who was looking into a harmful contraceptive implant called Essure, which is also the subject of Netflix documentary The Bleeding Edge. Read Lizzy's Q&A with Kinard here.
FDA seeks to regulate lab-developed tests
Also on the FDA, the agency announced plans late last week to start regulating tests developed in laboratories — an attempt to close what industry watchers call the "Theranos loophole," which likely allowed the inaccurate tests hawked by Elizabeth Holmes' ignominious venture to slip through regulatory cracks.
The FDA's latest proposal would add lab diagnostics to its purview, which is likely to draw support from patient advocates and ire from laboratories, Lizzy writes. Last year, Congress almost passed a bill that would give FDA that authority, but it faced opposition from Republicans arguing on behalf of academic medical centers and hospitals. At the time, FDA head Robert Califf said the agency would take action if the bill failed.
"Despite the important role [lab-developed tests] play in patient care, some of these tests perform poorly or don't work at all," Jeffrey Shuren, who leads the Center for Devices and Radiological Health at the FDA, said last week. "Because [lab-developed tests] aren't generally coming to the FDA for review, nor are additional safeguards in place, patients cannot be assured their tests provide accurate results." Read more.
Startups Digital health's deal count and funding slows
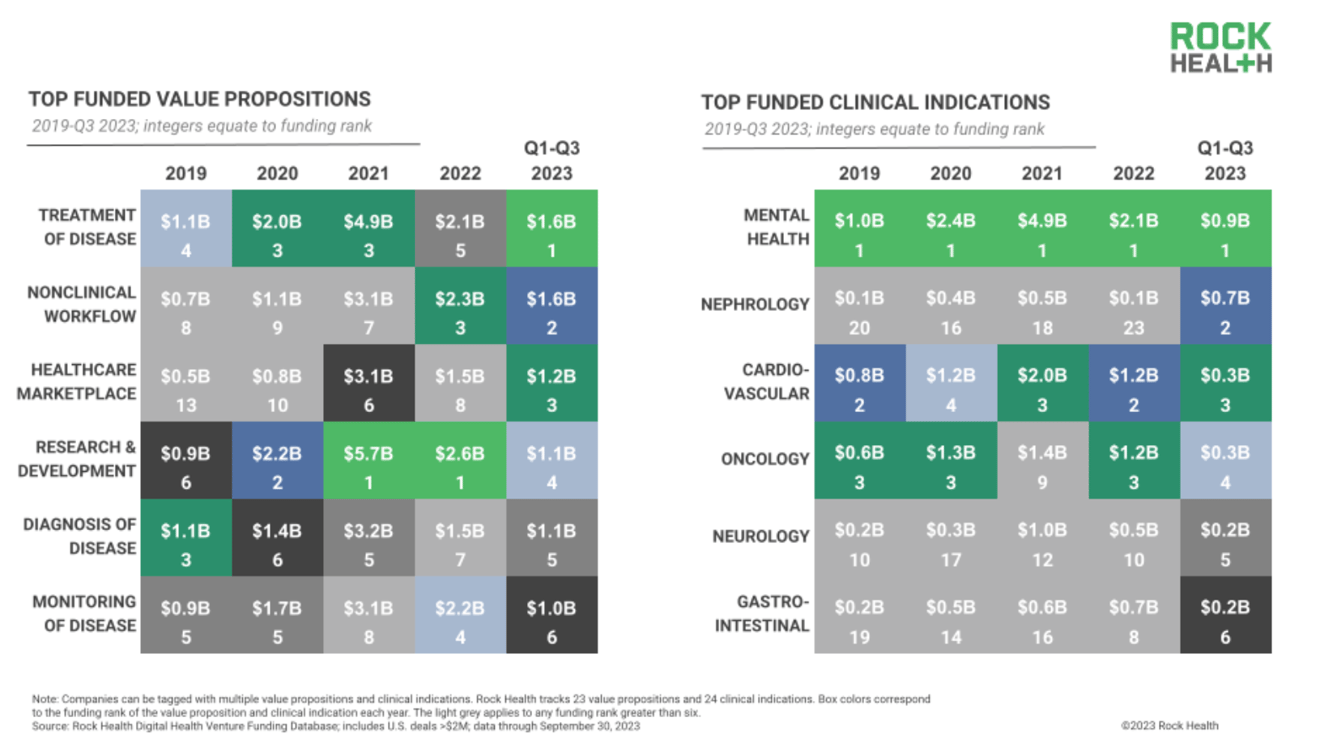
Rock Health's latest quarterly analysis expounds on a familiar theme we're likely to see for quarters to come: Digital health deals and funding are shrinking, but investors are still pouring lots of money into health care. U.S. digital health companies raised $2.5 billion across 119 deals, which amounts to the second lowest volume since the last quarter in 2019.
Looking at the past few quarters, it appears that investors are especially enthusiastic about ventures using technology to treat disease, and to make non-clinical workflow, like managing the distribution of durable medical equipment, more efficient. And mental health ventures are still taking home a large chunk of the venture checks.


No comments