closer look
'FDA has to be flexible': Janet Woodcock reflects on more than 30 years at the agency
Chip Somodevilla/Getty Images
It's no exaggeration to say that when Janet Woodcock leaves the FDA, it's the end of an era. She joined the FDA's biologics division in 1986 but soon moved to the drug side, where she oversaw hundreds of reviews, watched new therapies evolve, and weathered clashes with Congress and patient advocates. She took a look back with STAT's Sarah Owermohle.
What's different now for the agency?
More and more people are questioning authority and even authoritative scientific opinions, and the FDA is a part of that. It's all the regulators, it's all the authorities, it's the people in universities and everything. FDA has to be flexible.
What do you mean by 'flexible?'
It needs to be an agency that can react appropriately to societal trends, to recognize those and to be able to respond to them within its parameters, its authorities, its role.
How would you answer critics of accelerated approvals?
Some people might say there were some that didn't work out. Well, that's how accelerated approval is set up. Is that wrong? You hold back all of these until you have definitive evidence? Based on the results, you would have a lot of people who wouldn't be alive. Was that worth it to have all that certainty?
Read the full interview.
pharma
New Vertex pain drug stirs optimism and questions
Early yesterday morning, Vertex Pharmaceuticals shared late-stage trial results for a pain medication showing it was safe and effective while offering modest pain relief. But there was a catch, as STAT's Jonathan Wosen reported: The small molecule drug VX-548 didn't do better than a combination of acetaminophen and the opioid hydrocodone, a key secondary endpoint, for patients who took it after a tummy tuck or bunion surgery. And in the bunionectomy study, the opioid comparator beat VX-548 on pain relief.
The results set off a fresh debate about whether the drug would succeed, should it win approval. Pain experts and market observers pointed out that any new non-opioid pain medication would reverse a long history of pharma failure in this field, but wondered if payers might be reluctant to cover the drug for treating moderate to severe acute pain — where the company plans to seek first approval — without clear and consistent evidence the drug works as well as opioids. Jonathan has more.
reproductive health
Babies are arriving sooner, with a bump at 37 weeks
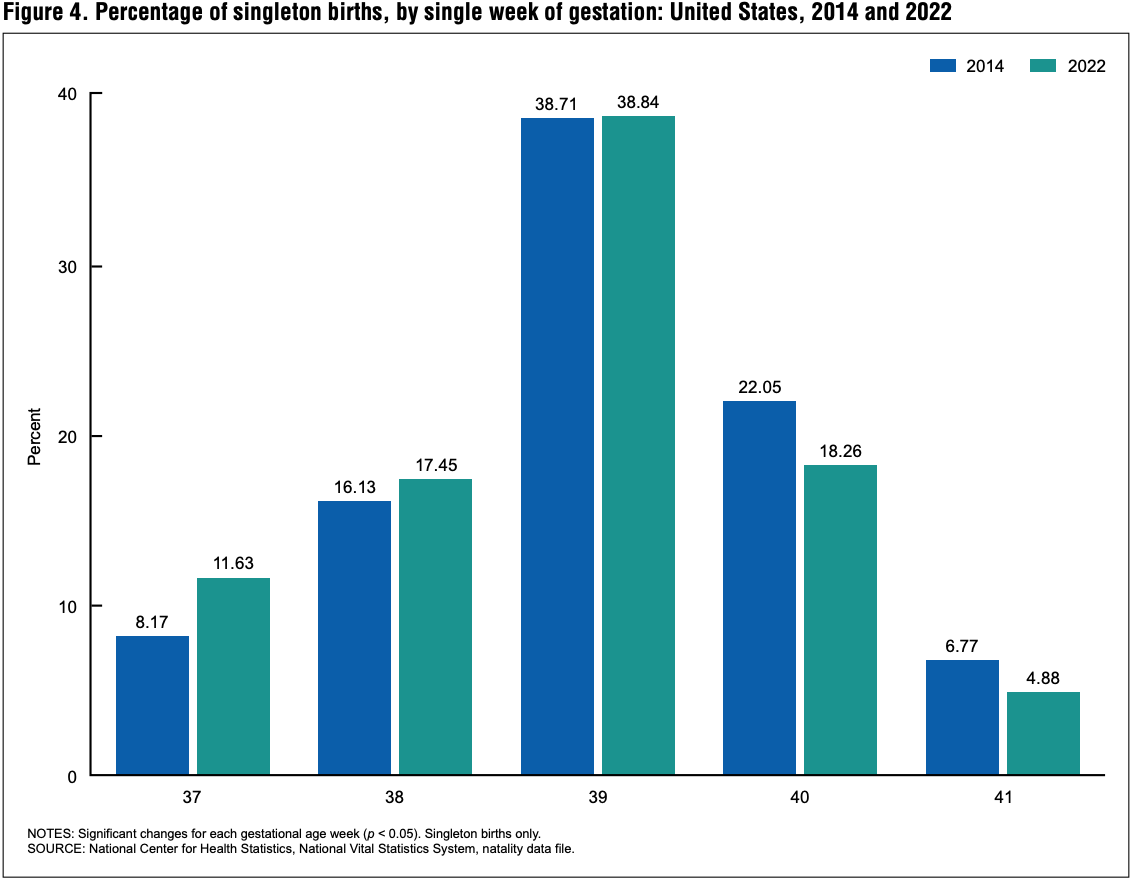
National Center for Health Statistics
Looking back to 2014 and allowing for pandemic fluctuations from 2020 through 2022, a new National Center for Health Statistics report tracks a shift toward singleton births after shorter gestational ages. While preterm (before 37 weeks) and early term (37 to 38 weeks) birth rates climbed by 12% and 20%, respectively, full-term (40 weeks) and late and post-term birth rates fell, by 6% and 28%, respectively. These trends were similar across maternal age, race, and Hispanic origin. The largest change for a single-week gestation at term was an increase of 42% for births at 37 weeks.
Some history: Preterm birth rates in the U.S. rose by more than one-third from 1981 to 2006, sparking concern and focusing attention on the risks for babies born between 34-36 weeks of gestation, or late preterm. These late preterm births accounted for 70% of all preterm births over that time period, today's report notes.


No comments