Venture capital
Startup investors eye security, privacy technology
Hospital corporate investment arms and Silicon Valley venture firms alike are nosing around for companies focused on privacy and security that could eventually support the other health-related AI startups they're already backing, experts told me last week. As my colleagues and I have reported, health systems have been rushing to deploy products like ambient documentation tools even before they've had a chance to establish benchmarks for quality, and cybersecurity risks, exacerbated by new technology, have soared.
Instead of waiting for federal and state regulators to catch up, investors tell me they're trying to get ahead of potential pitfalls by building out AI infrastructure — a step that could not only safeguards today's products, but could also help cement privacy and safety standards in an emerging industry that doesn't quite have them yet. "While the establishment of comprehensive regulations is still underway, it provides a unique opportunity for us to lead by example," said Jeffrey Jones, senior vice president of product development at UPMC Enterprises. Read more here.
General Catalyst, a16z health AI venture raises $53M
Health AI startup Hippocratic AI — backed and launched by both General Catalyst and its rival venture fund Andreessen Horowitz —has raised a $53 million series A round co-led by Premji Invest and General Catalyst, the company announced at the NVIDIA conference Monday. The latest round brings the LLM company's total funding to $120 million.
Hippocratic, which partners with several health system co-developers, also pushed out its first product for safety testing: a marketplace for generative AI tools that can do non-diagnostic patient-facing tasks.
Also at the NVIDIA conference, ambient documentation startup Abridge announced an investment from the computing giant's investment arm, NVentures. Abridge also plans to use NVidia computing resources and models to drive its own generative AI technology, the companies said this morning.
Survey: Consumers won't share data with just anyone
An annual consumer poll from venture seed fund Rock Health finds that the majority of those surveyed in 2023 — 90 percent — were willing to share health data with at least one outside entity, spanning from clinicians to insurance companies to health tech companies. But, interestingly, the proportion of people willing to share their data with each entity type has dropped in recent years — 70 percent of respondents were willing to share their data with clinicians in 2022, compared with 64 percent in 2023, for instance. And there were significant variations in willingness to share data by age, race and ethnicity. "[C]onsumers' growing reluctance to share their healthcare data is an opportunity and a warning for the sector," report authors wrote. "There's an unmistakable need to address concerns and invest in trust-building around data sharing and stewardship."
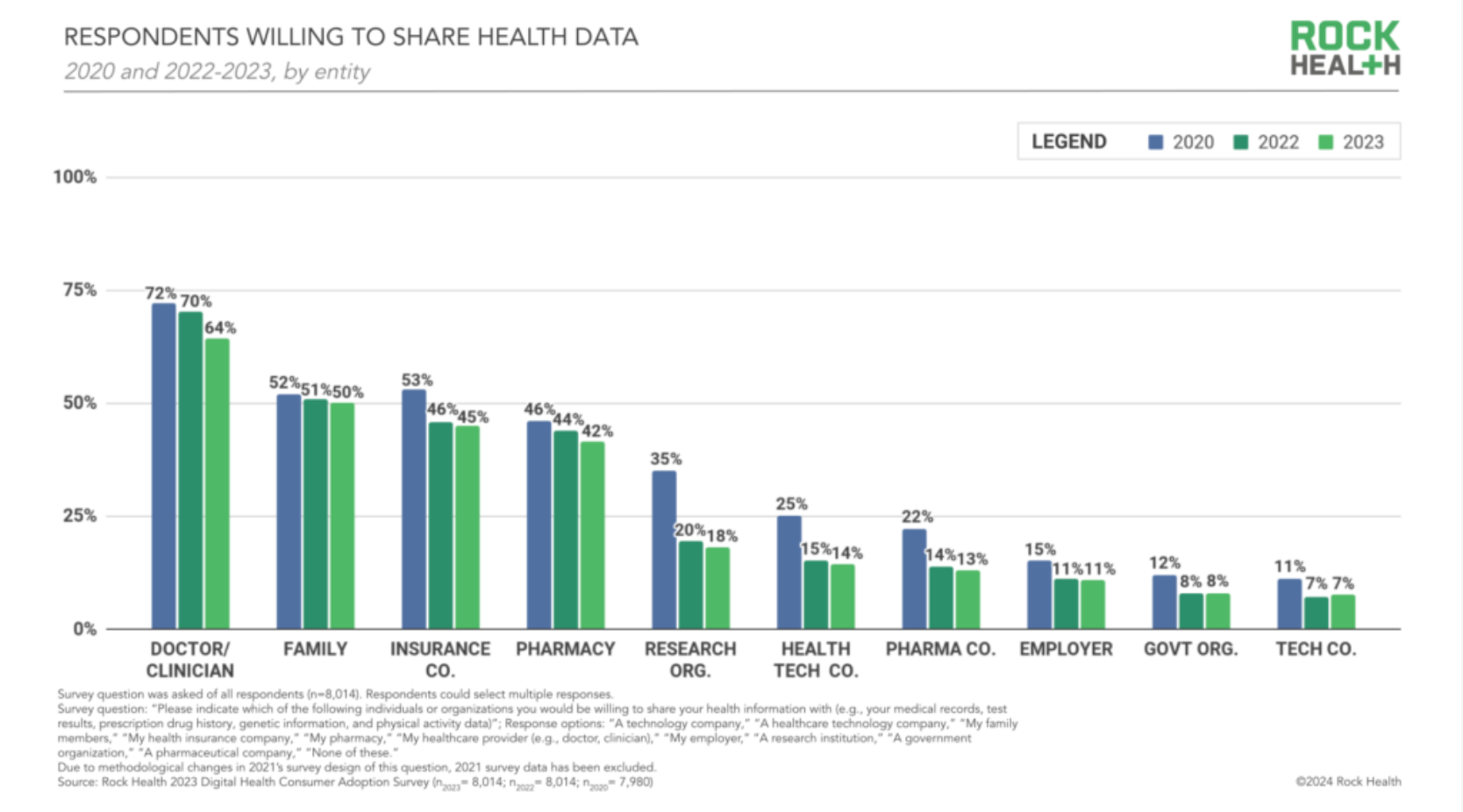
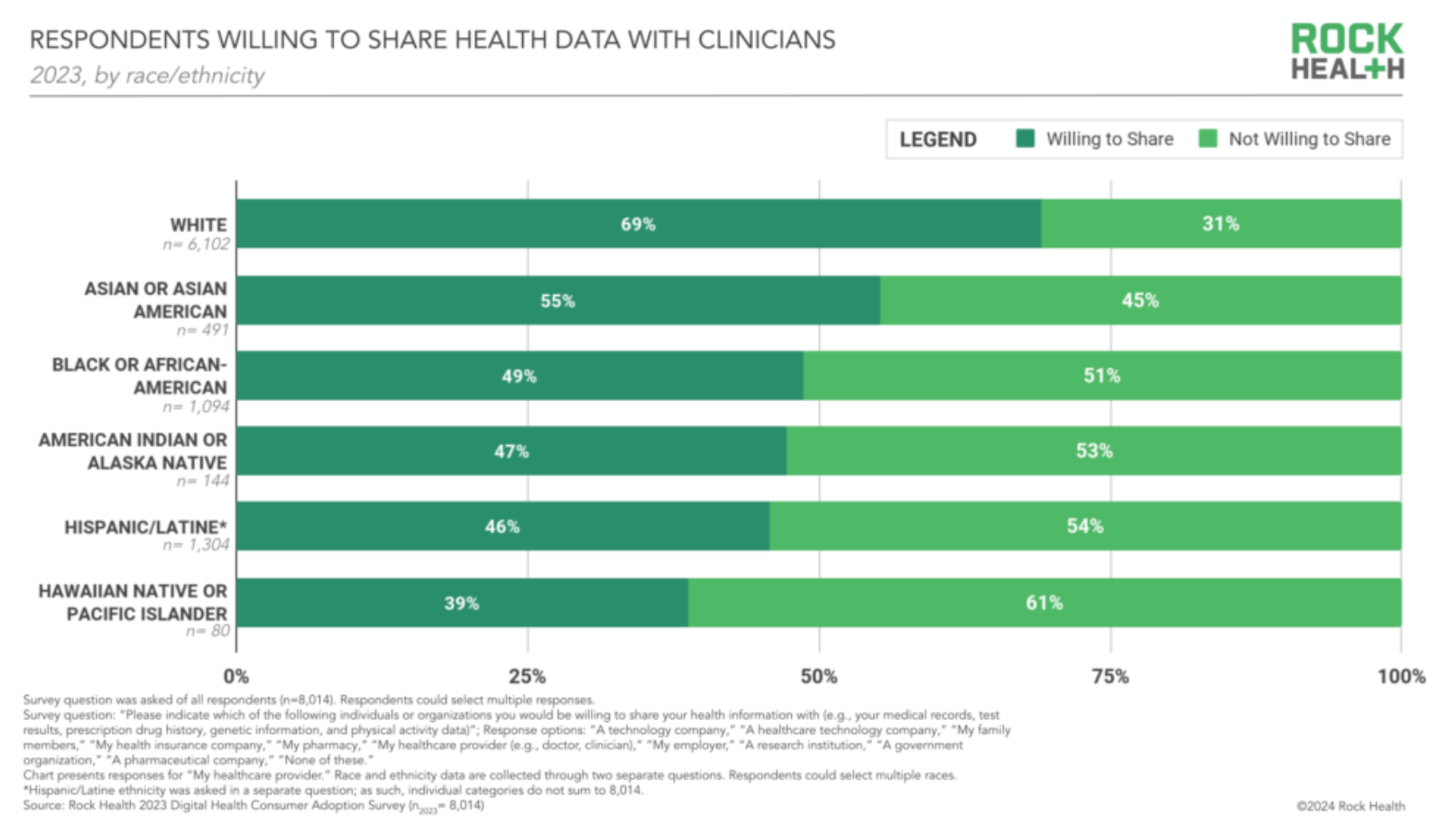
White respondents had the highest proportion willing to share data with clinicians; Hawaiian Native and Pacific Islanders had the lowest. Medical racism and historical discrimination, of course, contribute to these gaps — but I welcome any theories underlying consumers' growing reluctance to share health data with clinicians.
Academia
Study: Direct-to-consumer telehealth linked to higher rates of antibiotic receipt in pediatric patients
With mountains of usage data amassed since the pandemic's early stages, experts are beginning to chip away at some of telehealth's foundational questions — including whether the modality alone changes the care patients get. Staunch telehealth advocates have argued vociferously that it doesn't, but a new study in JAMA Network Open paints a different picture, albeit only in one specific context: pediatric patients receiving antibiotics.
In an analysis of about 28,000 pediatric patients with acute respiratory tract infections, researchers found that virtual visits associated with primary care providers were less likely than those with direct-to-consumer telehealth companies to result in antibiotics prescriptions, a diagnosis warranting antibiotics, or a follow-up visit or fulfilled prescription within two weeks. About 29 percent of primary care provider telehealth visits resulted in antibiotics, compared to about 37 percent for direct to consumer companies, the study found.
The data offers a small window into potential differences, but questions remain: It's still not clear whether any differences are due to the "modality of care (telemedicine vs in-person) [or] the context of telemedicine care (primary care vs not primary care)," authors wrote. Supporting telehealth visits through primary care providers could be one way to reduce the receipt of antibiotics, they noted in the study.







No comments