FDA
An AI company is scanning genital pictures for STIs
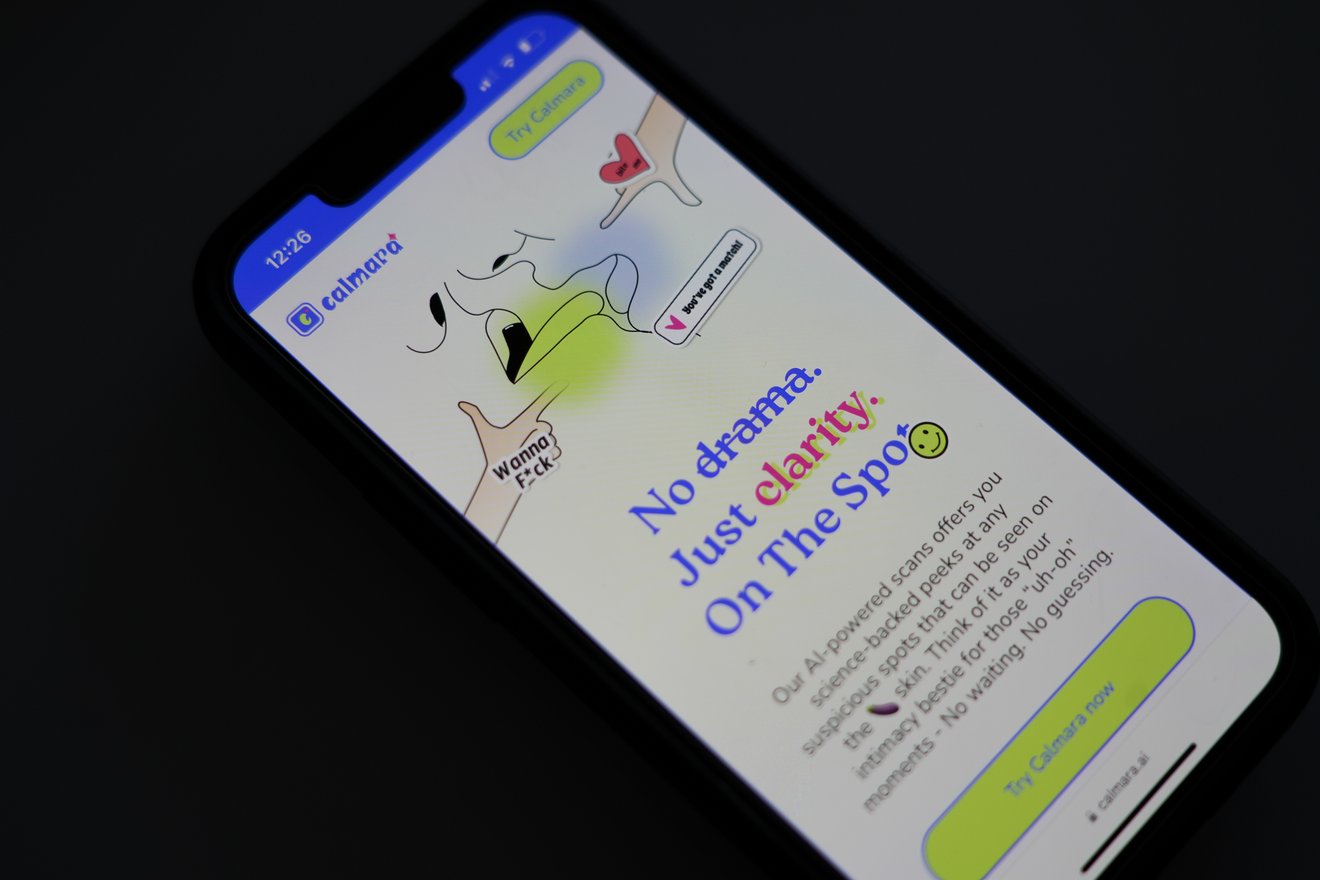
This week Lizzy Lawrence takes a closer look at a company generating outrage and bemusement over its online platform that uses AI to detect sexually-transmitted diseases from genital photos. The company, HeHealth, recently launched a new service called Calmara that it has so far attempted to market to Gen Z women who might want to establish the absence of STIs before sex.
Questions and criticism, of course, abound — especially from privacy experts, patient advocates and medical practitioners who point out that the platform appears to sanction nonconsensual photos, and that there's little proof that the AI detection even works. And the creators could be taking advantage of a regulatory gray area that allows the platform that self-categorize as "wellness" products to evade Food and Drug Administration scrutiny.
"If I were still at the agency, I would give them a call and a 'it's come to our attention' email,'" Alex Cadotte, a director at device consulting firm MCRA and former FDA official, told Lizzy. Read more.
Otsuka's digital depression treatment cleared by FDA
In other regulatory news, the FDA has cleared a digital treatment for major depressive order developed by Otsuka Pharmaceutical, Mario Aguilar writes. The prescription product, branded as Rejoyn, was developed in conjunction with Click Therapeutics, and is designed to be used alongside antidepressant medication. It's a six-week program focused on "cognitive-emotional training," which asks users to identify and recall faces displaying various emotions — the technique is supposed to train users to exert cognitive control over "emotional information processing." This step, Mario writes, makes Otsuka the first drug company to score FDA clearance for digital mental health treatment; but prescription digital services are far from cash cows, and several companies have already run themselves into the ground trying to build them. Read more from Mario.
cybersecurity
Could Change Healthcare bring on new cyber rules?
Washington regulators are in the very, very early stages of strategizing to avert future cybersecurity attacks like the one that crippled UnitedHealth Group property Change Healthcare's pharmacy and provider payment system, disrupting billing across the country for weeks. It's too early to say what the new regulations could look like — that'll depend on the election, how quickly Congress and executive branch agencies can move, and how much pressure industry groups exert on policymakers — but they'll have major implications for hospitals, pharmacies and tech vendors, experts told me last week.
"How quickly can people step back from being angry about what happened to Change, and realize, 'let's really think about the roles — the roles are, are you the victim? Or are you the criminal?'" Rodney Whitlock, a vice president at McDermott+Consulting, told me. Read more.
artificial intelligence
Inside the White House's AI plan for government
A White House memo posted late last week outlines sweeping new policies for federal agencies' use, and governance, of AI services — including a directive that all agencies name chief AI officers and spin up AI governance boards. Over the next several months, agencies must also "implement concrete safeguards when using AI in a way that could impact Americans' rights or safety," among other steps.
Federal health care systems, such as the Veterans Health Administration, would need to closely examine any AI-guided diagnostic tools and ensure that "a human being is overseeing the process to verify the tools' results and avoids disparities in healthcare access."
How useful are these guidelines, and how burdensome might it be for federal agencies to implement them? I invite your perspectives at mohana.ravindranth@statnews.com.


No comments