Venture Capital
Q1 2024: Deals grew while checks shriveled
Digital health funding appears to be picking up pace, though checks are shrinking — 2024's first quarter tallied the lowest total quarterly digital health venture funding since 2019, but racked up 133 deals, which topped each of the last six quarters, according to a new analysis from seed firm Rock Health. "Investors and founders are finding their stride and closing deals at more measured check sizes than in previous quarters," authors wrote.
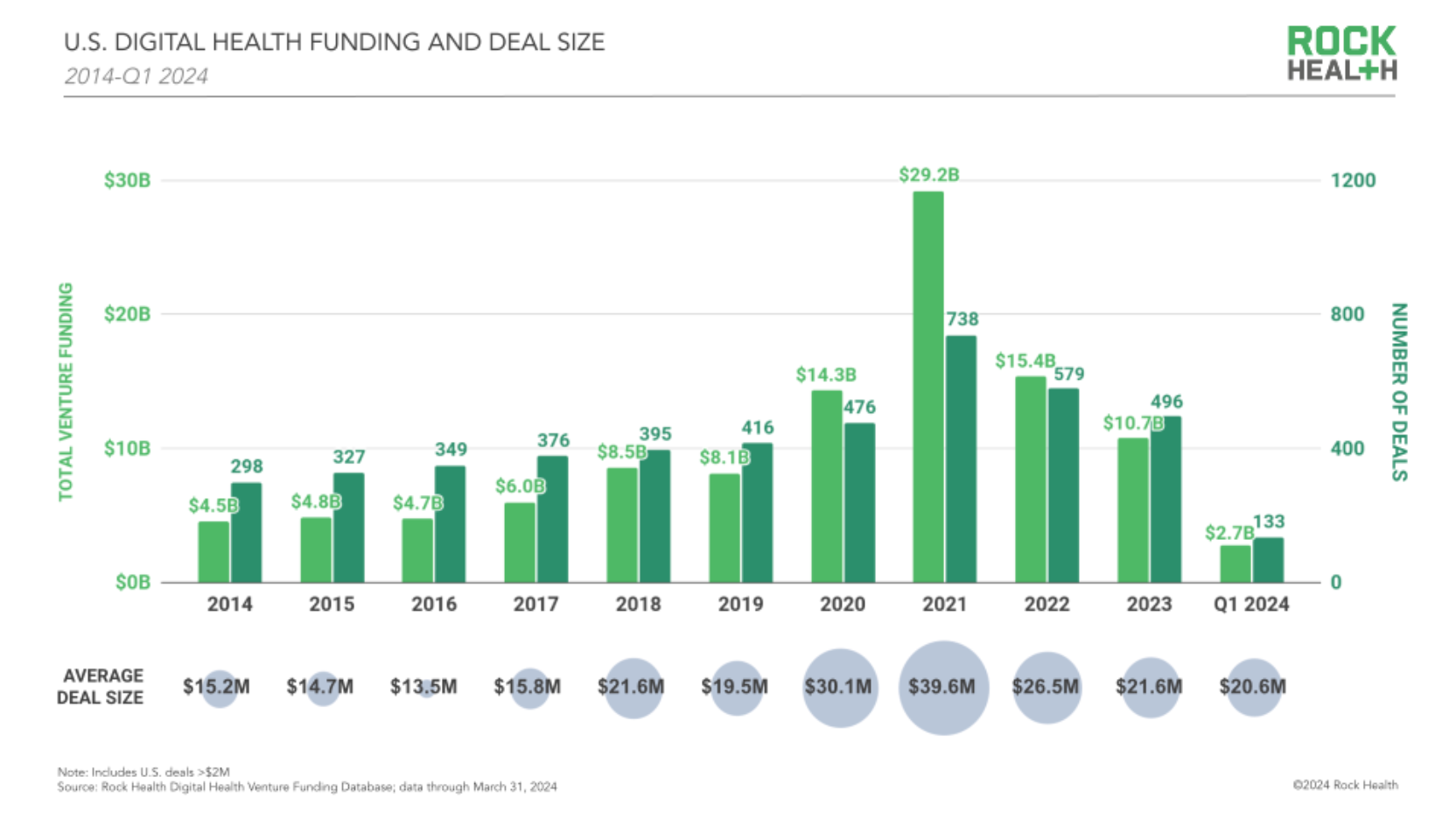
The industry is also seeing a lot more "creative fundraising," which includes unlabeled funding for companies that aren't ready to raise — about 48 percent of Q1's deals fell into that category, authors wrote. And companies hawking AI services took home 40 percent of total digital health funding, up from 33 percent across 2023. Read more.
Hospitals
Inside Providence's sweeping innovation plan
I sat down with health system Providence's chief digital officer Sara Vaezy, who oversees the health system's innovation arm that recently spun out its fourth tech business Praia Health, a platform health organizations use to link their patients to health services online. She told me Providence does some of its own software development, makes financial investments in promising outside technology, and also incubates nascent ideas into full fledged companies — a multi-pronged strategy for getting the best new technology into clinicians' and patients' hands, Vaezy said.
In some instances, she said, health systems must realize they simply can't build better products than outside vendors can — and in others, it makes most sense to build a custom product in-house. But partnerships and contracts with outside groups, whether it's tech companies or venture firms funding other tech companies, are "gaining momentum" this year at Providence, she said. "There's so much innovation happening in the market, we can't outcompete it," she said. Providence is also a member of the Coalition for Health AI, a trade group working alongside government on setting standards for clinical uses of AI.
Asked how she decides when to partner externally instead of building a product in-house, she said there's no exact roadmap — but that Providence is going "all-in" on disparate innovation programs.
"We participate in multiple consortia, we participate in multiple different activities. We have our own corporate venture fund, we are a limited partners in a variety of other venture funds, we also do our own build. Our approach is super pluralistic: it really depends on the domain, the thesis area, which mechanism is most appropriate."
virtual care
Teladoc CEO Jason Gorevic departs immediately
The longtime leader of major public telehealth company Teladoc has abruptly stepped down from his post, surprising analysts and raising questions about the firm's viability as a potentially profitable business. In a statement late last week, the company said Jason Gorevic would depart immediately, and that chief financial officer Mala Murthy will act as his replacement in the interim.
Despite skyrocketing telehealth use during the pandemic earlier stages, the company has experienced high-profile stumbles, including its acquisition of chronic care company Livongo that netted billions of dollars of impairment charges. Still, Gorevic oversaw the business' revenue growth from $553 million in 2019 to more than $2 billion in 2021, my colleague Mario Aguilar writes. Read more from Mario.
Medical Devices
Few who are eligible for cochlear implants get them
Though they're widely known for addressing hearing loss, cochlear implants are far from accessible to the general public — and especially to marginalized communities including Black and Asian patients, my colleague (and STAT's inaugural disability in health care reporting fellow) Timmy Broderick writes. According to a study of British hospitals in PLOS Medicine, most patients who met the threshold criteria for implants weren't referred, and there were significant disparities by socioeconomic status, sex and ethnicity.
"If, as clinicians, we're not presenting them with that information, then they don't have a chance at knowing [that] this life changing intervention is an option for them," said study co-author Chloe Swords, an otolaryngologist at the University of Cambridge. Read more from Timmy.
J&J makes $13.1 billion deal to buy Shockwave
Johnson & Johnson said late last week that it planned to buy heart device company Shockwave Medical — the pharma and med tech giant's second major cardiovascular deal in recent years, following its $16.6 billion acquisition of Abiomed in 2022, Lizzy Lawrence reports.
Shockwave's flagship device is designed to use sonic waves to break up plaque in coronary and peripheral arteries; the company says it has treated 400,000 using this treatment.
Clinical trial offers some support for Shockwave
A clinical trial fully independent of Shockwave has gathered some data supporting the company's heart device's use, according to data presented on Monday at the American College of Cardiology's annual meeting. But though the device reduced chest pain associated with heart disease, it didn't work using the mechanism researchers expected it to: forcing blood back through the heart's veins.
"We like to understand why things work or don't work, and when things are biologically plausible and there's a clear mechanism, they're more likely to get embraced," said Yousif Ahmad, a Yale interventional cardiologist said. Read more on researchers' academic dilemma from Lizzy.
Inside Abiomed heart pump's first big clinical trial
A long awaited randomized clinical trial's results also were revealed at the American College of Cardiology conference this weekend, suggesting that a controversial heart pump from Abiomed cut down on deaths in severe heart attack patients. The findings were also published in the New England Journal of Medicine.
The trial followed more than 300 patients for six months in Denmark, Germany and the U.K. — those enrolled had come into hospitals with a heart attack and low blood flow. Half of those patients got standard care, usually medication or life support, and the other half got Abiomed's heart pump, sold as the Impella. About 46 percent died in the device group, compared to 59 percent in the standard care group. Read more from Lizzy.






No comments