artificial intelligence
Using AI to diagnose Mendelian disorders
An intriguing new artificial intelligence approach to diagnosing inherited diseases was just outlined in NEJM-AI. The tool, called AI-MARRVEL, trained on 3.5 million genetic variants from thousands of Mendelian disorders. With a precision rate of 98%, it correctly identified 57% of diagnosable cases out of 871 cases.
"The diagnostic rate for genetic disorders is only about 30%, and on average, it is six years from the time of symptom onset to diagnosis," Pengfei Liu, one of the study's authors and a researcher at Baylor University, said in a statement. "There is an urgent need for new approaches to enhance the speed and accuracy of diagnosis."
The tool does have several limitations, however: While it can process single gene mutations and small insertions or deletions, it isn't yet able to analyze structural variations or copy-number variations. But the authors say that it has the potential to discover novel disease genes — and think AI-MARRVEL could ultimately be used by clinicians and researchers to identify and interpret many genetic variations.
return on investment
Deloitte report: What's the value of an approval?
There's been a boost in the return on investment for pharma R&D, a new Deloitte report shows. In 2023, ROI rose by 4.1% — a substantial leap from the sector's low of 1.2% in 2022, and a pandemic peak of 6.8% in 2021. That said, it's still stymied by patent cliffs, a shapeshifting regulatory system, and how complex and quickly evolving science is becoming.
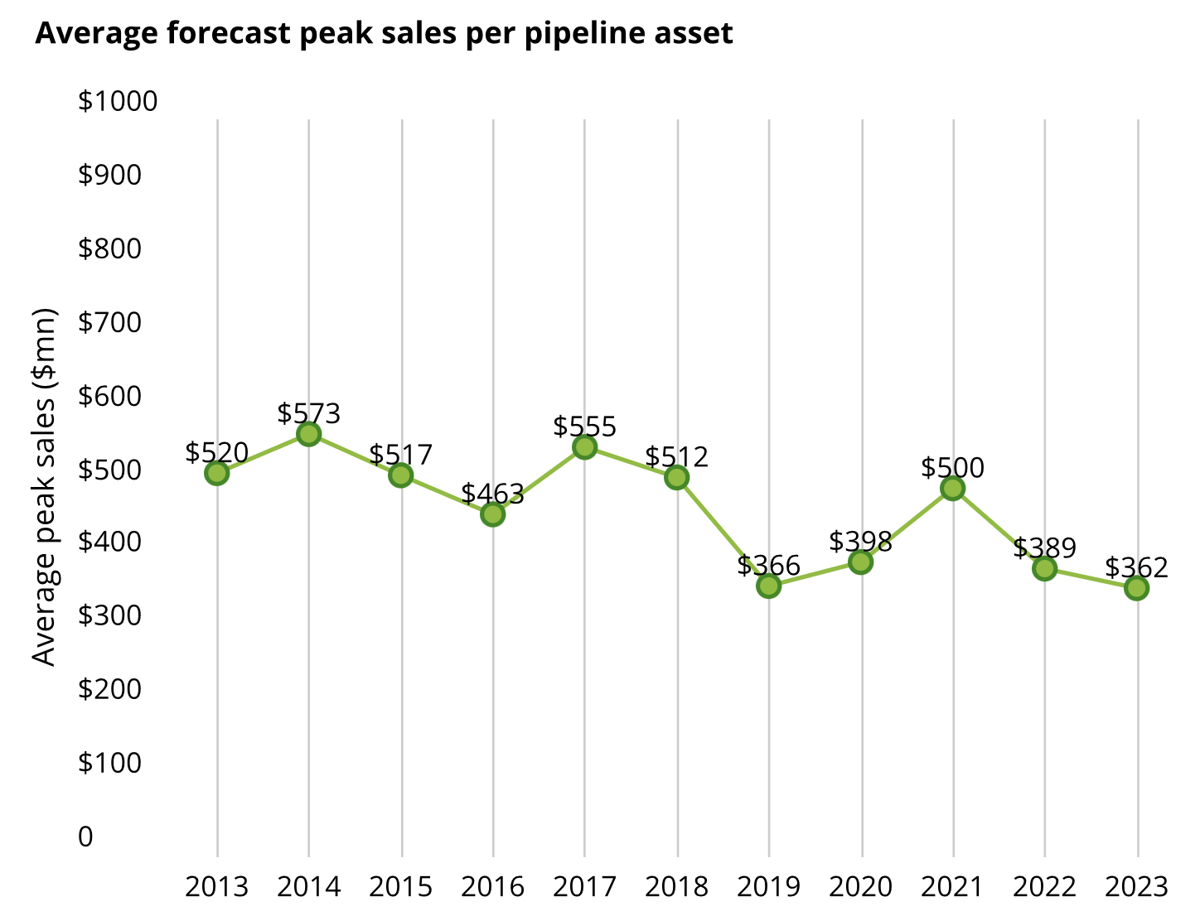
The average R&D cost to develop an asset from discovery to launch seems fairly level. But with the exception of that Covid-19 sales boost in 2021, there hasn't been improvement in terms of peak sales per asset, the Deloitte numbers show. Which does stir up an ever-looming question: What's the true financial value of an approval today?


No comments