insurance
How UnitedHealth upended a medical network
For months, my colleagues have been investigating how UnitedHealth has stealthily built up a massive physician empire and exploited its growing power to milk the system for profit. In the latest in their ongoing series, they zoomed in on one primary care network in Connecticut.
About 10 years after United acquired the group, doctors are retiring or leaving earlier than they planned. Patients with serious medical conditions struggle to make appointments, while others complain of mysterious diagnoses popping up in their charts.
"We used to always have 25 openings at the beginning of every day for people who are sick. And if you called our practice, we would see you today, always," one doctor said. But after United's Optum took over, "when our patients called, there weren't openings for weeks and weeks and they were told to go to the emergency room."
Read more.
glp-1s
Hims takes a blow from Lilly's vial news
Share in Hims & Hers, one of the most prominent telehealth companies offering compounded GLP-1 drugs, sunk over 7% yesterday. This came after Eli Lilly launched vials of its obesity drug Zepbound, which had previously only been offered in the U.S. as injectable pens.
Patients have increasingly turned to cheap, compounded GLP-1 treatments as the branded options carry a high price tag and have fallen into repeated shortages. One way Lilly is trying to combat that is with its new vials, which are priced lower than the list price of Zepbound pens and are easier and faster to manufacture.
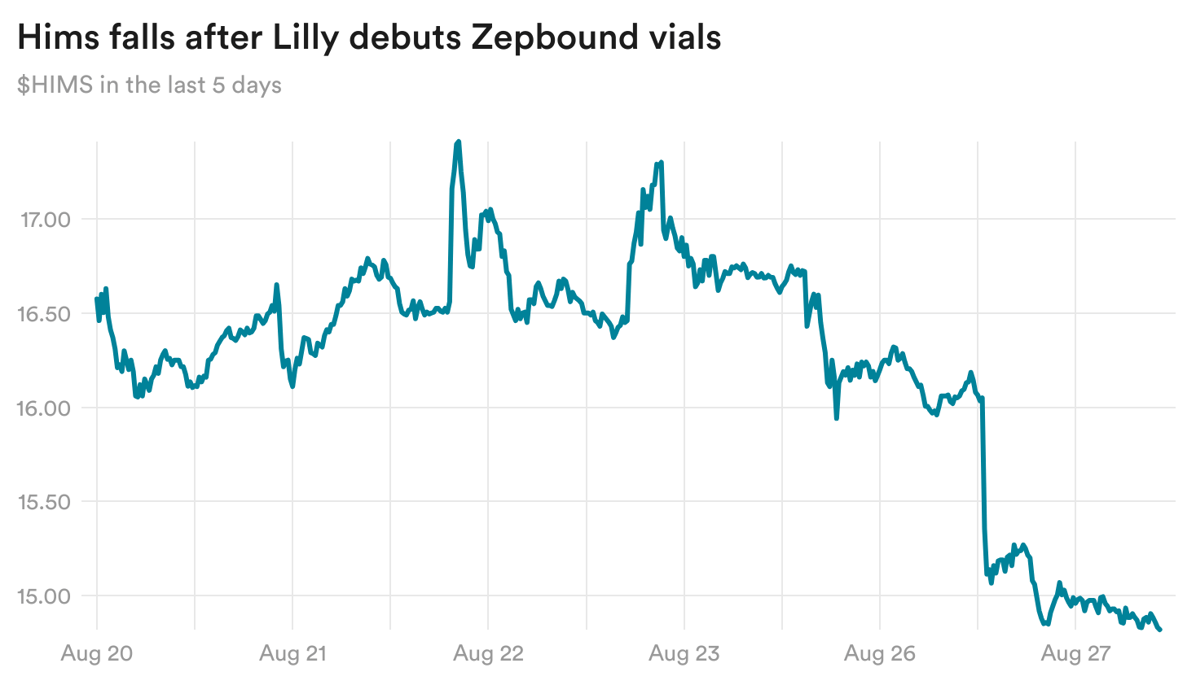
Despite how investors have responded, it's not clear if Lilly's new offering will actually dissuade patients from going to compounders. The Zepbound vials are priced up to $549 a month, while Hims offers compounded options as low as $199 a month.
Elsewhere, Lemonaid Health, a telehealth provider owned by 23andMe, said today that it's also started to offer compounded GLP-1 drugs — a further indication that telehealth companies will not be deterred from the market, even as pharma giants like Lilly threaten legal action against some players.
policy
Medicare's new coverage pathway doesn't go far enough, researchers say
Earlier this month, the Centers for Medicare and Medicaid Services unveiled a new pathway designed to offer early coverage for promising devices while investigating gaps in evidence of effectiveness and safety. In a new opinion piece, a group of researchers from the Center for the Evaluation of Value and Risk at Tufts Medical Center argues that this new pathway doesn't go far enough.
CMS should broaden the scope of the pathway to include not just devices, but also drugs, they say. They also argue that CMS should have more resources to collect real-world data on the new technologies, so that the agency can better assess their effectiveness and safety.
Read more.


No comments