Real ESTATE
There's too much lab space in Boston
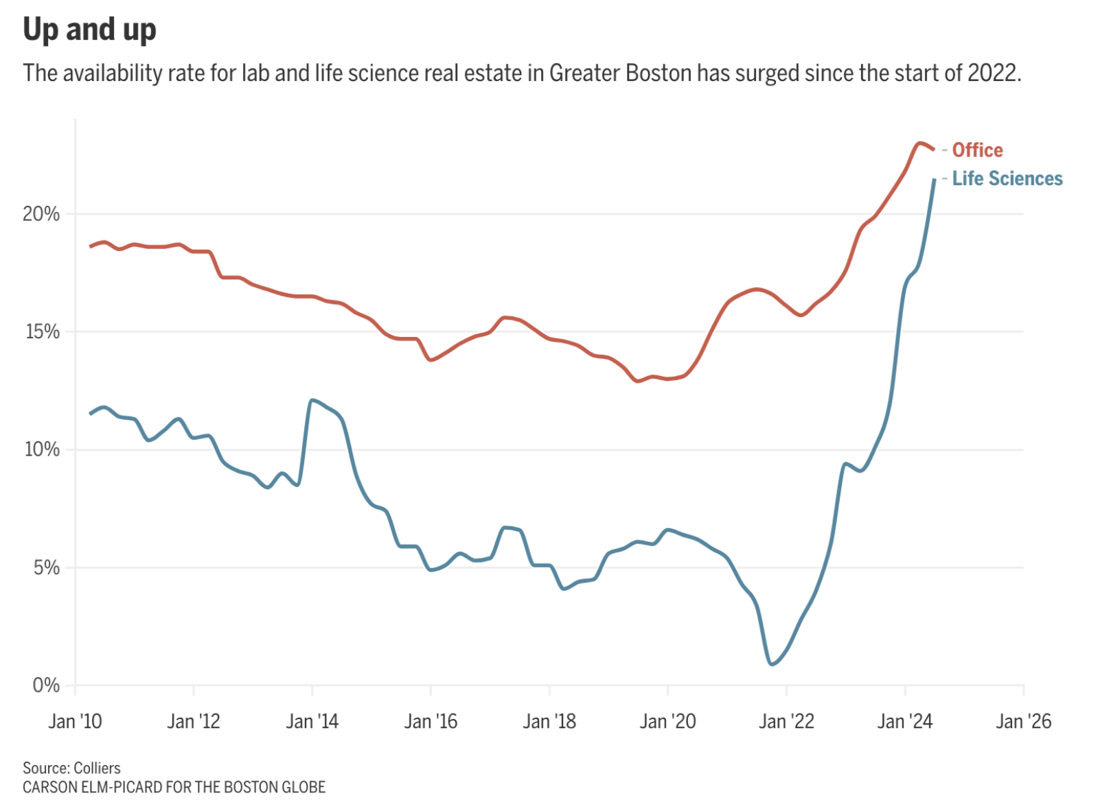
Demand for lab space during the Covid-19 pandemic spurred rapid real estate development in the Boston area — but now there's a tremendous oversupply. Right now, 21.5% of lab space there is vacant, compared to just 1% three years ago. This surge in vacancies is attributed to speculative building by developers, an influx of venture capital during the pandemic, and the broader slowdown of the biotech sector.
This amounts to about 11 million square feet vacant in the Boston area, with more expected as new projects are completed. While some look at this as a market correction, others actually look at it as a chance for smaller biotechs to grow.
Read more.
podcast
Is pharma still fearful of the Inflation Reduction Act?
Will Lykos' FDA rejection and recent publication retractions pull down the entire psychedelics industry? And what would you name a commercial psychotherapy treatment? We discuss all that and more on this week's episode of "The Readout LOUD."
STAT's chief Washington correspondent Rachel Cohrs Zhang joins us to discuss the Medicare drug price discounts and how this first round of negotiations between pharmaceutical companies and Medicare officials played out. After that, I join Elaine, Allison, and Adam to talk about some of the latest news in the psychedelics world, including the retraction of three research papers.
Listen here.
hiv/aids
Gilead's patent hopping with unsafe HIV drug
Gilead Sciences, which is a top HIV drugmaker, settled a $40 million lawsuit from 2,625 people who claimed they were harmed by the drug tenofovir disoproxil fumarate, or TDF. And a larger case in California, which involves 24,000 plaintiffs, accuses Gilead of delaying the release of a safer version of of the drug — saying the company is maximizing profits by patent hopping, or switching patients to the new drug only right before TDF's patents expire, despite knowing it's unsafe.
"When pharmaceutical companies do good work, they should be able to turn a decent profit," opines Peter Staley, cofounder of PrEP4All, an advocacy organization promoting a national HIV prevention plan. "But dragging their feet to bring a safer treatment to market while thousands of people are taking a harmful one isn't doing good work."
Read more.


No comments