exclusive
An unexpected offer to the 'CRISPR babies' scientist
He Jiankui, the researcher who created the first gene-edited babies, was approached by a crypto entrepreneur named Ryan Shea offering funding to start an embryo editing company, STAT has exclusively learned.
In some ways, Shea isn't who you might expect to see investing in altering the DNA of humans before birth. He has no biotech or medical experience.
But in other ways, Shea is exactly the kind of person you should imagine getting behind He's latest project, because of his vision for the future, one that's shared by some of the most powerful people in America.
"There's a growing interest among Silicon Valley types in shaping the future of the species," a biomedical historian said. "There's always been a transhumanist sensibility there — a sense that those who don't participate in the technological transcendence of the human will diminish, will remain in the evolutionary past and fade away. But increasingly, that's become less of a science-fiction fantasy sensibility and more of an investment opportunity sensibility."
Read more from STAT's Megan Molteni.
health tech
Biogen looks to telehealth to raise disease awareness
As Biogen aims to boost sales of its postpartum depression drug Zurzuvae, the biotech is turning to telehealth company Talkiatry to raise awareness of the condition.
Under a new deal, Talkiatry created a new landing page on its website with information about postpartum depression. From there, visitors can take an intake assessment that funnels them into Talkiatry's care programs. The Zurzuevae website will include a link to the landing page. The terms of the deal were not disclosed.
The partnership resembles efforts by pharma companies like Eli Lilly and Pfizer to team up with telehealth companies to make it easier to get their drugs.
Read more from STAT's Mario Aguilar.
biotech
Scholar Rock sees SMA data boosting obesity effort
Scholar Rock yesterday reported that its experimental treatment for spinal muscular atrophy succeeded in a Phase 3 trial, boosting muscle function in patients. Shares of the biotech surged over 360% on the news.
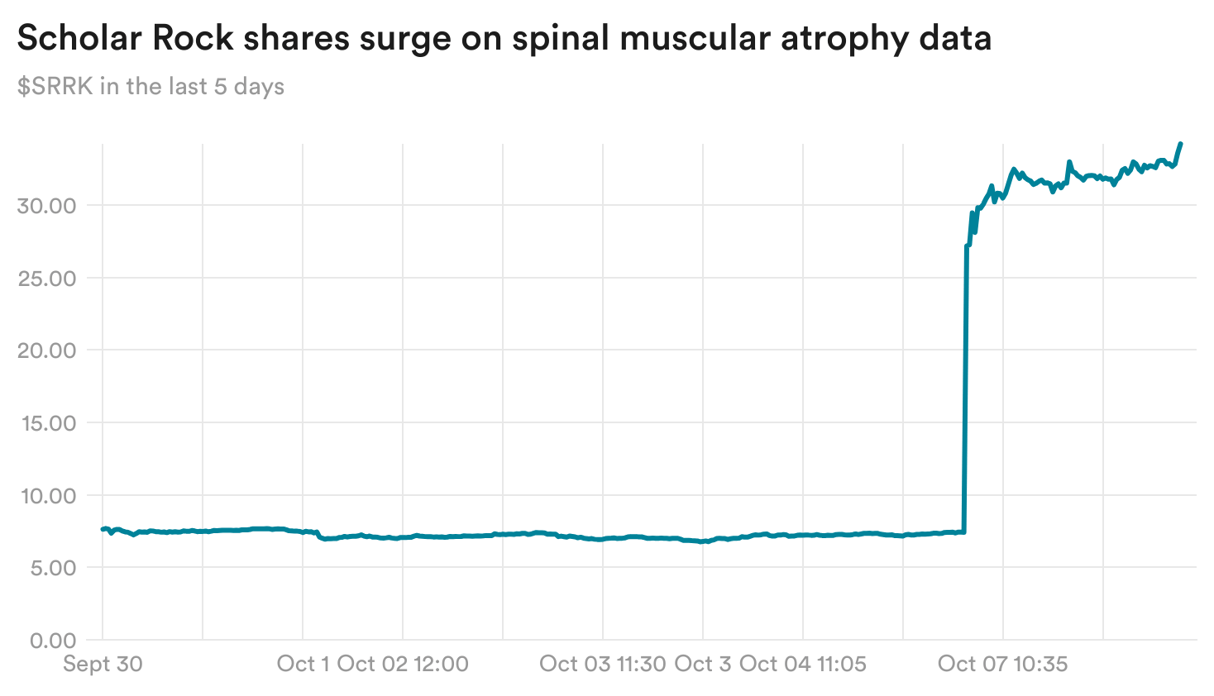
The company is also hoping that the mechanism behind the treatment, blocking a protein called myostatin, could be helpful in obesity by offsetting the lean mass and muscle loss that patients experience while taking weight loss drugs.
It's running a Phase 2 trial testing the drug, apitegromab, in obese patients who are also taking a GLP-1 drug. It's a proof-of-concept study that'll inform the development of a new myostatin inhibitor, called SRK-439, that's specifically focused on obesity.
Jay Backstrom, CEO of Scholar Rock, said in an interview that the new results are "further affirming" of his confidence in the obesity indication. What'll be interesting to watch is how the biotech prices the drugs, if they both eventually make it on the market, given that SMA is considered a rare disease and obesity is widespread.
"We're looking at apitegromab from the lens of orphan disease, and we'll be thinking about the pricing and reimbursement against that lens," Backstrom said. "And then because it'll be a separate program that'll be a few years down the road as we run the studies, [the new molecule] will be in more of that general medicine marketing."
Read more.
exclusive
Janet Woodcock's next steps after the FDA
Janet Woodcock, a former top drug regulator at the FDA, has joined the board of a patient group called Every Cure that's focused on scouring old drugs to see if any could be repurposed to help patients with rare diseases.
"I thought their approach was intriguing, you know, trying to map the pathways and then match them to extant products," said Woodcock, who retired from the FDA in January. "I don't believe they'll hit every time, but I think there are probably some gems out there that can help people that have already been developed and yet aren't being used or tested in that disease."
Woodcock said in an interview that, for the most part, she's not taking positions on boards. She's not considering roles with for-profit companies, both because of the potential perceived conflict and because she's not sure those companies need her help. Her role on the Every Cure board is unpaid.
Read more from STAT's Matt Herper.


No comments