Artificial Intelligence
How HHS is using AI
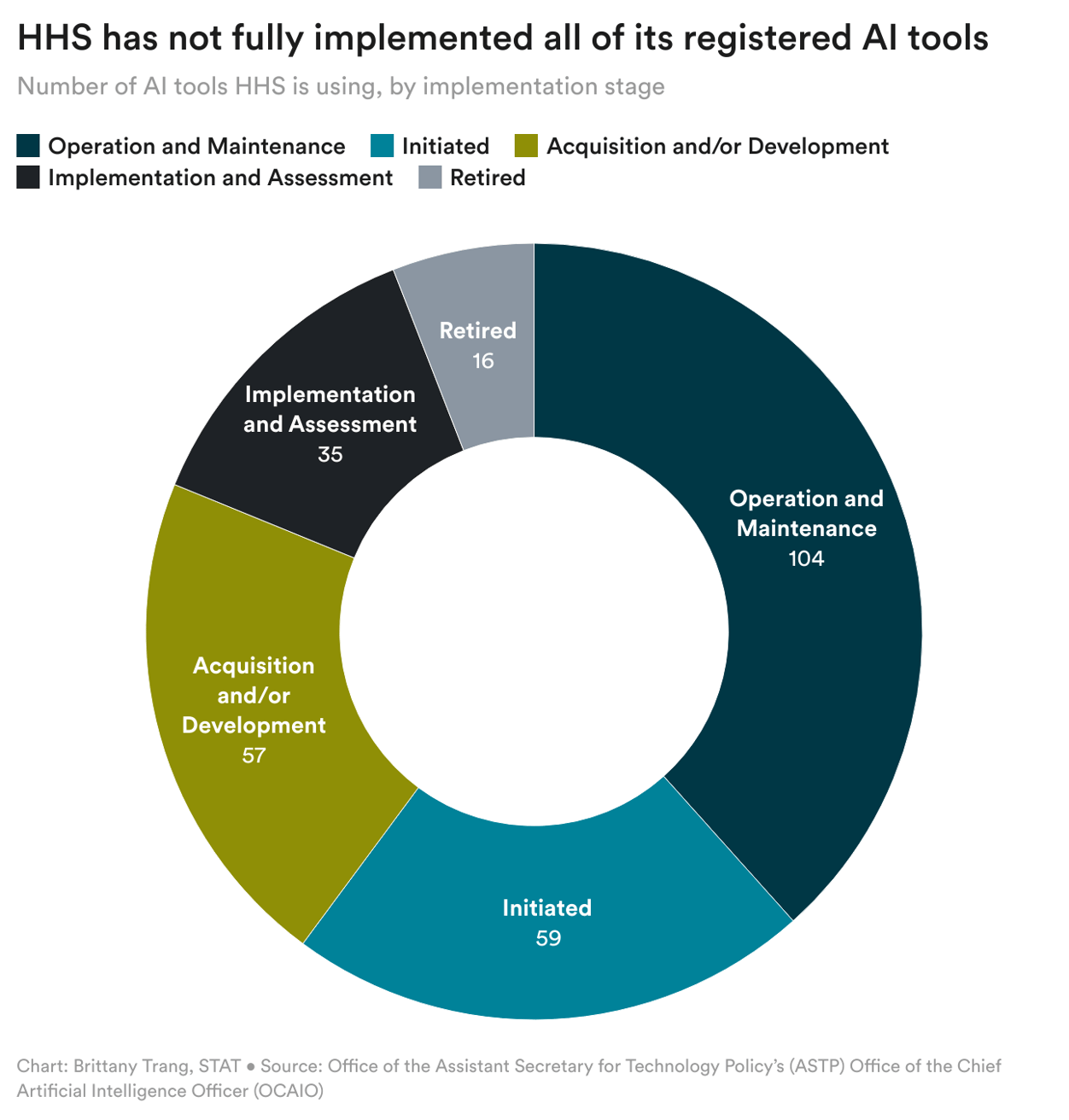
Yesterday the Department of Health and Human Services released its AI Use Case Inventory, which details the AI tools the agency is using internally.
The federal agency's AI use cases range from Microsoft Word autocorrect, to a nascent "HHSGPT," to identifying sewage facilities from photos. Many center around text-based tasks such as helping sort comments on proposed rulemaking, de-duplicating and querying adverse event reports, or searching various internal text data collections with natural language processing.
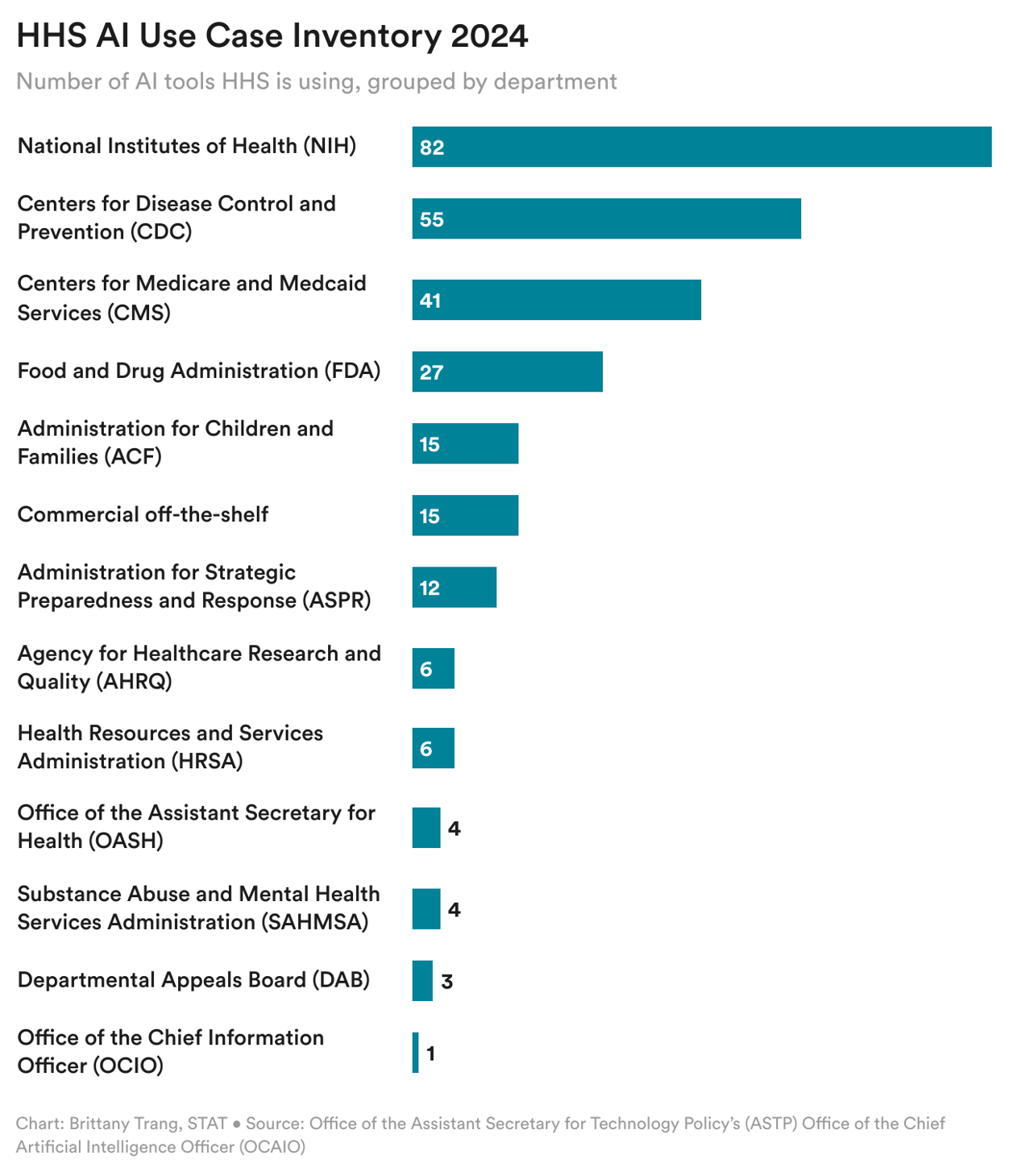
Note that, as shown in the chart above, not all of the tools are yet fully implemented, and some have been "retired" already. The agency disclosed 271 tools this year, a 66% increase from 163 last year. As seen in the chart below, the NIH, CDC, CMS, and FDA account for over 75% of the tools (though all but three of the 16 retired tools were from CDC and NIH.)
A couple of use cases that stood out to me:
-
Speaking of fighting AI with AI, CMS has initiated a tool called "Medicare Part C/D Plan Oversight of AI Used for Prior Authorization and Utilization Management" that identifies outliers in claims, payments, and complaints data to determine if health plans may be out of compliance with CMS rules. (If using AI tools to deny care in Medicare Part C plans, aka Medicare Advantage, doesn't sound familiar, make sure to check out STAT's Denied by AI investigation.)
-
The Office of Refugee Resettlement (ORR) Unaccompanied Children Bureau has initiated the use of "process digital twins to provide the ability to run 'what if' scenarios" in its mission of temporarily caring for and releasing unaccompanied refugee minors. ORR's goal for the tool is to "be more proactive in strategic planning and contingency planning."
Medical devices
Children aren't just little adults…so why do AI medical devices think they are?
In a new JAMA Pediatrics study, researchers found that 22 AI and machine learning-enabled medical devices that are FDA authorized for kids were validated on adult data alone. And, 99 of the AI/ML devices approved for use in children omitted data indicating whether they were tested in children.
"There have been many studies where tools have been trained on adults and then tested for performance on pediatrics, and they've all done substantially worse on pediatrics," said Marla Sammer, vice chair of clinical affairs at Texas Children's and chair of the American College of Radiology's pediatric AI workgroup, who wasn't involved in the research.
STAT's Katie Palmer has more on the study, the hurdles involved in testing devices for children, and why this can lead to worse outcomes for kids.
STARTUPS
Funding for brain-computer interfaces
Brain–computer interface company Precision Neuroscience yesterday announced the closing of a $102 million Series C funding. The company plans to use the funds to further test and develop their implant, which enables people with paralysis — such as severe spinal cord injuries or ALS — to operate digital devices with their thoughts. While their timeline for bringing the technology to market remains vague, "this funding brings us one step closer to that vision," said Michael Mager, the company's co-founder and CEO.
The BCI field is at an interesting moment. A recent Morgan Stanley report valued the market at $400 billion, and as Elon Musk becomes a top advisor to President Donald Trump, there's an assumption that investors and federal officials will provide the industry with the space and capital to grow.
However, multiple sources suggest that BCI companies, like biotech startups, are capital-constrained. Precision's original Series C filing in October suggested that the team had set out to raise as much as $150 million, but a company spokesperson said the goal was $100 million and that their legal counsel put down the larger number "to give us latitude."
Stay tuned for more news about the BCI industry, as companies race to be the first product on the market. —Timmy Broderick


No comments