policy
Trump rolls back artificial intelligence order
Following President Trump's inauguration, our eyes are on sweeping changes he's pushed through executive orders, and as expected, he rolled back former President Biden's order on artificial intelligence from 2023. At the time, STAT's Casey Ross did a rundown of what the Biden EO meant for health AI developers. The best-known provision required developers of foundation models "that may pose a risk to national security" to provide information to the federal government, including about their safety testing. This was incredibly unpopular with industry.
The order also made a number of demands of the Department of Health and Human Services, including that it develop a strategic plan for artificial intelligence, which it only just delivered, and an inventory of ways the department makes use of AI. Despite the recent excitement around AI, the federal government and Congress did not make much progress on guardrails around the technology, in health care or otherwise, though regulators made some limited rules. It's unclear what exactly the Trump administration will do, but it's certain to be a more hands-off approach.
resarch
A look at marketing for compounded GLP-1s
GLP-1s are the hottest drugs in a generation, and if you have eyeballs, you've been bombarded with marketing about the popular obesity and diabetes drugs' tremendous benefits. But are you sure everything you're reading online is true?
The drugs are expensive, demand has led to shortages, and online services have rushed to fill the void with lower-cost compounded copies. As STAT's Katie Palmer reports, a new study found that of 79 websites marketing compounded GLP-1s or prescriptions for them, 37% stated or implied that the drugs were FDA-approved. Nearly half didn't include information about the drugs' adverse effects, warnings, and contraindications. Compounded copies are not approved by FDA.
The study underscores concerns about the proliferation of compounded GLP-1s, which may or may not be on the down swing as shortages ease and as drug companies consider ways to offer the brand-name drugs at lower cost.
Read more here
policy Model card madness
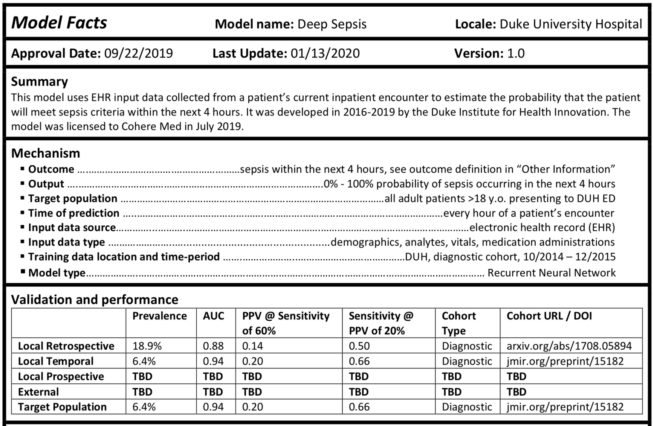
Following a federal deadline at the end of the year, developers of certain AI products used in health care must provide end-users a number of details about how they were developed and how they should be used. The use of "model cards," or "nutritional labels," to detail machine learning and other technologies is widespread in the broader tech industry, but its use in health care is newer. So to help developers comply with the new rules, or simply to help them disseminate information about their products, the Coalition for Health AI and the Duke Institute for Health Innovation have each published free-to-use templates.
Interestingly, though the Food and Drug Administration hasn't actually mandated that developers of regulated AI medical devices detail the products with model cards, its draft guidance document for AI developers released earlier this month says model cards can be useful and includes an example. Among the concerns of industry is that dueling regulatory regimes will make compliance difficult, so it will be interesting to see what efforts are made to harmonize recommendations across the federal health department.


No comments