markets
Biotech's strong news day wasn't enough to support the XBI
It's been a long time since we experienced such a positive biotech news day like yesterday:
Beam Therapeutics's CRISPR treatment showed encouraging results in a debilitating lung condition. Mineralys Therapeutics said that its experimental drug significantly reduced blood pressure in two different studies. Protagonist Therapeutics reported positive results of the drug it's developing with Johnson & Johnson in ulcerative colitis. And Checkpoint Therapeutics, a cancer immunotherapy company, said it would be acquired by Sun Pharma at a 66% premium to its previous closing price.
You'd think this amount of good news, and stock moves for the individual companies (Protagonist surged 46% yesterday), would at least offer the XBI temporary reprieve from its prolonged slump. Yet the industry was still weighed down by the broader market.
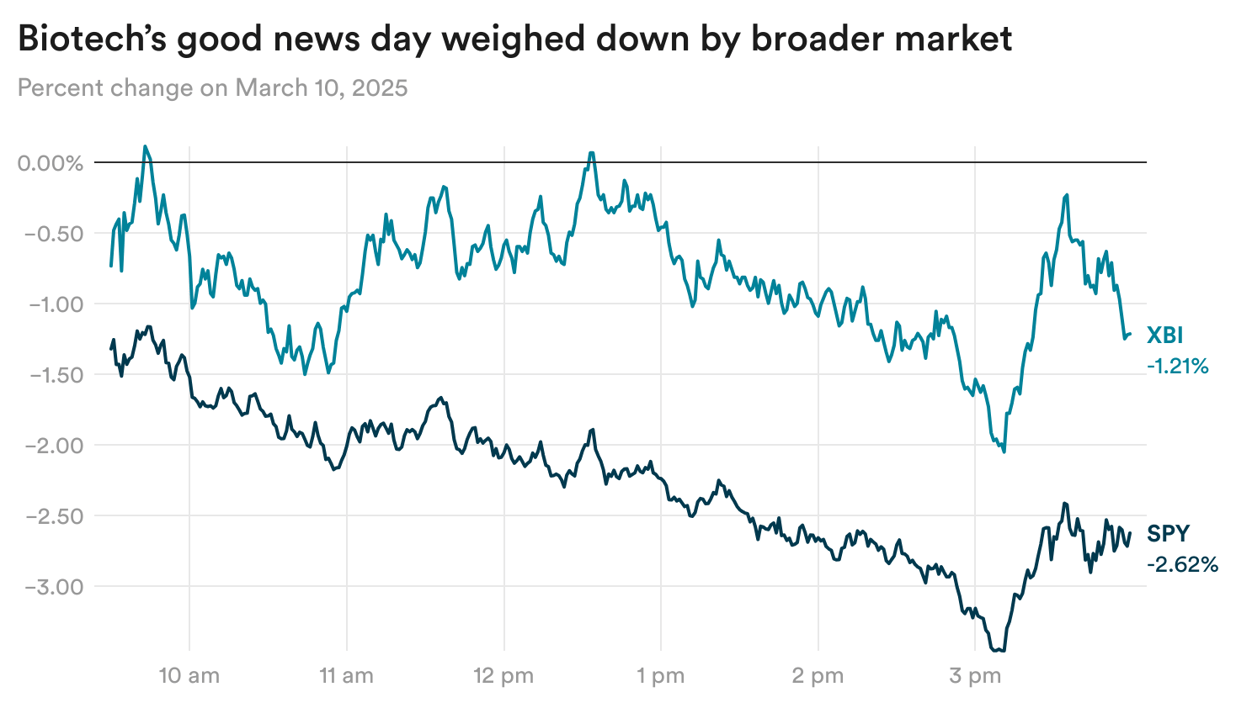
U.S. stocks saw their biggest drop of the year yesterday, with the SPY falling 2.6%, as President Trump declined to rule out a recession as a potential result of his economics policies. The XBI outperformed the SPY, but still fell 1.2%.
politics
FDA blocks reviewers, inspectors from buyout offer
Yesterday, we brought you the news that HHS sent a buyout offer to workers across its departments, including the FDA, CDC, and NIH.
Endpoints News reported that the FDA told staff that reviewers of drugs and medical devices and inspectors of manufacturing facilities cannot take the buyout offer. Many of these staff are funded by user fees paid by drug and device sponsors to ensure the timely review of their products.
This is the latest in a turbulent series of developments for FDA staff. Last month, the Trump administration fired FDA employees as part of a government-wide cost-cutting effort overseen by DOGE, but then rehired some of the workers days later.
Health tech
Telehealth companies pivot from GLP-1s to hormones
As branded GLP-1 drugs fell into shortage over the last two years, hundreds of telehealth companies popped up to prescribe compounded copies. But as branded GLP-1 drugs come back into supply, threatening the business of compounded copies, dozens of telehealth companies are now shifting to offer hormone replacement therapies.
Noom, best known for its weight loss app and more recent GLP-1 offering, launched a hormone therapy program for menopause in late February, and telehealth company Hims & Hers plans to roll out at-home testing over the next year to enable care for low testosterone, perimenopause, and menopause.
Patients have long advocated for better access to hormone-based care. But clinicians and health policy researchers are concerned that the commodification of hormone therapies — often marketed as a personalized fix for low energy, libido, and other age-related concerns — could lead to inappropriate prescriptions and put patients at risk.
Read more from STAT's Katie Palmer.
pharma
How well can a pharma trade group regulate its members?
The Association of British Pharmaceutical Industry, a U.K. trade group that sets industry code, is due to lift a rare suspension it imposed on Novo Nordisk for "serious breaches" in promoting an obesity drug.
Has Novo learned its lesson, though? Since the suspension, the company again breached various codes for infractions such as promoting an obesity drug to the public through sponsorships at pharmacy chains and failing to disclose substantial payments to health and patient groups.
Given that, academics question whether the public should feel reassured by the industry group that Novo has substantively changed its business practices and that the group is properly enforcing its own rules.
Read more from STAT's Ed Silverman.
obesity
Investors yet again disappointed by Novo data
And speaking of Novo — the Danish company's shares plunged again yesterday, as investors were disappointed by new data of the next-gen obesity drug CagriSema in patients with diabetes.
In a late-stage trial of people with obesity and type 2 diabetes, patients on CagriSema lost 13.7% of their weight over 68 weeks when looking at all participants, including those who dropped out.
Investors were expecting greater weight loss, particularly since an earlier mid-stage trial of CagriSema showed that patients with obesity and diabetes lost 15.6% of their weight over a period of just 32 weeks. In this new late-stage trial, though, Novo incorporated a flexible dosing protocol, meaning it allowed patients to modify their dosing rather than stay on the highest dose.
Read more.


No comments