fda
Former FDA chief: Loss of generics team jeopardizes progress
Recent layoffs at the FDA threaten to undo a cornerstone of the Trump administration's efforts at improving drug affordability: the fast-tracking of generics. In a new First Opinion in STAT, former FDA commissioner Scott Gottlieb points out that the agency recently dismissed 13 veteran staff from its Division of Policy Development — experts who wrote the scientific playbooks that allowed lower-cost versions of sophisticated drugs like Ozempic and COPD inhalers to reach the market.
"The FDA's political leaders reconsidered cuts initially made elsewhere within the agency's drug programs, even rehiring some key scientists," he writes. "However, the regulatory policy team in the generic drug group was dismissed wholesale, with no plans to restore this uniquely specialized team."
While some generics giants can go it alone, most smaller firms rely on this guidance to compete, he writes. The move, framed as a cost-cutting measure, is short-sighted and could undo years of progress, Gottlieb says.
Read more.
clinical trials
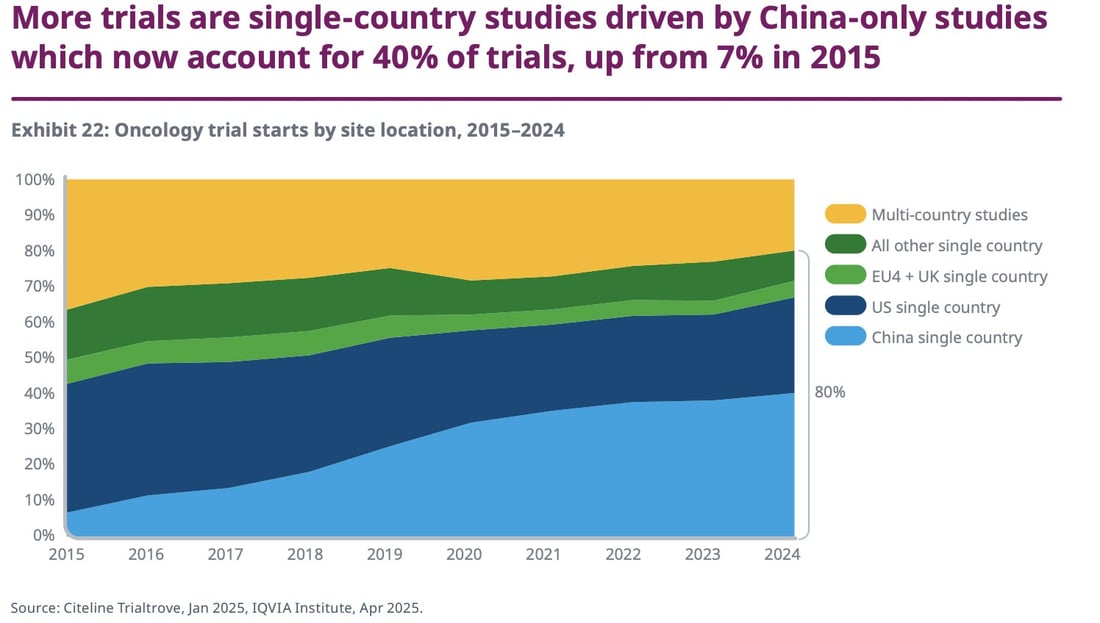
More biopharma trials are happening only in China
A global oncology report from IQVIA outlines, via cancer trial stats, how China's role in biopharma is ever-increasing. There are fewer studies being conducted across several countries, while more are being conducted in China — which could spell a downtick in trial diversity. In 2015, 37% of trials were multi-country studies, compared to just 20% in 2024. Meanwhile, China-specific studies rose from around 5% in 2015 to around 40% in 2024. Behold:


No comments