Just in
And the National Coordinator for health IT is...
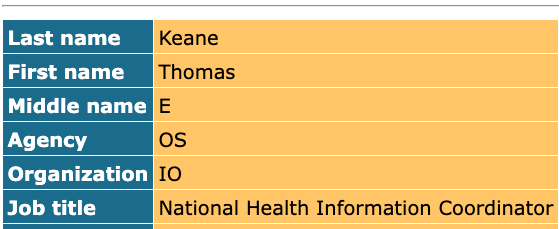
We heard from two sources plugged into the world of health IT that Thomas Keane had his first day as National Coordinator for Health Information Technology yesterday. The federal health department staff directory appears to confirm Keane's new role, but we haven't heard anything official from the department.
Keane is a radiologist who previously served as an advisor to ONC. Traditionally, this position has been the health department's top health IT regulator, overseeing policies around interoperability and electronic health records.
Now, given the reorganization at HHS, the role is in flux. Under the Biden administration, ONC's purview was expanded, and it became the Assistant Secretary for Technology Policy. But according to HHS' newly released budget request documents, ASTP/ONC will now report to the HHS chief technology officer who will also oversee cybersecurity. (More on this document below.) What this means for the future of ONC's regulatory power and funding is unclear.
Artificial intelligence
'Rushed' FDA AI takes flight
The Food and Drug Administration yesterday announced a broad rollout of Elsa, an agency-wide generative AI tool meant to help staff work more efficiently. Earlier in the day, STAT's Brittany Trang broke the news that Elsa was coming. Her story includes way more detail about development behind the scenes including a sense among agency employees that the rollout is being rushed.
Read more here
Health it
Medicare's ambitious tech push
Mehmet Oz's Medicare agency is plotting a very ambitious tech modernization agenda. Among those driving the effort: Amy Gleason of the U.S. DOGE Service and Arda Kara, a senior advisor for technology at CMS. In a new story I spoke to them a bit about their plans, which are in early development.
A big takeaway: They want to move fast to launch something to show concrete progress in 2025. They also told me about some high-level ideas under consideration, their approach to deciding what to tackle first, and about how they want to stimulate innovation in the private sector.
On cue, the day I published the story, CMS issued a document spelling out how it plans to spend its 2026 budget, if approved by Congress, including several details on tech modernization plans (See pages 105 to 111):
- Reengineering claims systems and using AI and automation to transform claims processing. "These technologies will drive greater efficiency, enhance payment integrity and enable a more scalable and resilient system architecture," CMS writes.
- Launching an AI lab that "will support the deployment of AI-powered virtual assistants and chatbots across support centers and deliver workforce training in prompt engineering and applied AI."
- Consolidating many CMS data repositories, which CMS says "enables seamless data sharing across the healthcare ecosystem, provides a foundation for advanced analytics and AI applications, improves data quality and consistency, and enhances program integrity through comprehensive data visibility."
Many big ideas. If you want to chat about any of them, please reach out. And read my whole story on CMS modernization here.
policy Eye-catching tech details in the HHS budget document
It wasn't just CMS: The entire federal health department published budget request documents on Friday spelling out priorities. A few health tech details caught my eye.
- FDA: Under accomplishments of the office of the commissioner from 2024 that are in line with current priorities, FDA cites the Digital Health Advisory Committee meeting hosted in November, which focused on regulating generative AI. A key staff member who drove this effort left the agency this year, and there are questions about what would become of the committee itself, and whether any readout would be published from the meeting. I read its inclusion in the budget document as a signal that the work may continue. (Pages 66 to 67 of FDA's budget justification.)
- Health IT: In addition to hints about ASTP/ONC, federal health department also explained that it will continue TEFCA, the national health data sharing framework. (Page 41 of the HHS Budget in Brief document.)
- NIH: As STAT reported Friday, the documents detail the scope of budget cuts coming to NIH. As a bonus item, I wanted to flag this interesting request for information on responsibly developing generative AI tools for research.


No comments