INvestment
Will recent IPOs help digital health funding?
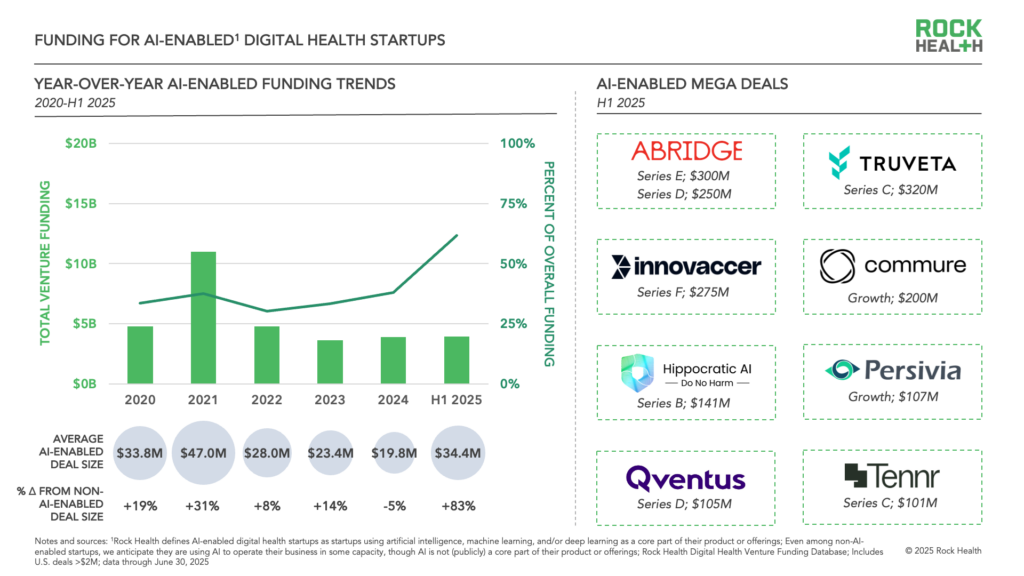
Digital health startups raised $6.4 billion dollars in the first half of 2025, a modest increase from the the same period a year before, according to an analysis from Rock Health. AI-enabled startups accounted for 62% of the funding; the money overall was raised across fewer deals; and there have already been 11 "mega deals" over $100 million — we saw 17 all of last year. (Two of those mega deals were for AI scribe company Abridge.)
The Rock Health authors go on to make the case that though the top line investment number didn't move much from recent years, the first half gave us proof points illustrating the potential of digital health. First, we saw Hinge Health and Omada go public, illustrating that "tech-enabled services companies are realizing transformed, sustainable care delivery." And second, the speedy adoption of well-funded AI use cases like AI scribes "show that breakout growth is possible with the right mix of provider trust, intuitive and impactful products, and capital to scale."
Will these proof points help drive a big second half of the year? We'll see.
Julie Yoo from a16z on "challengers" and "enablers"
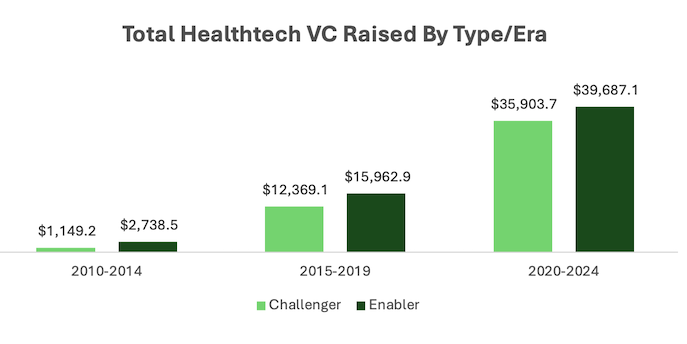
Andreessen Horowitz general partner Julie Yoo analyzed nearly 3,800 health tech VC deals from 2010 to 2024 and found a recent relative increase in funding for "full stack" companies that have cropped up to challenge incumbent players. These challengers, or "insurance companies, medical services companies, pharmacies," are contrasted to "enablers," like startups working on AI automation and revenue cycle management. From 2010 to 2014 enablers raised more than double the capital compared to challengers. From 2020 to 2024, it was just 10% more. Over the last 15 years, "Enablers have dominated in terms of overall volume of deals, but the ratio of VC deals into Challengers versus Enablers has risen," Yoo writes.
The analysis draws on a similar dynamic from the venture-backed tech industry where both challengers like Airbnb and Uber and enablers like Stripe and Databricks were needed to prop up the transformation we've seen today. Yoo's whole post goes much deeper on the two kinds of startups and also offers a link to the raw data.


No comments