business
Digital health for truckers
Truckers, on average, only live to the ripe old age of 61. Diabetes rates are twice as high as in the general population, and it's not uncommon to hear about drivers who die behind the wheel. It's a somber reality that inspired physical therapist Mark Manera to found a digital health company focused on the particular needs of a life lived on the road.
In building Offshift, Manera has confronted the realities faced by many entrepreneurs trying to build and sell lifestyle programs to prevent and manage chronic disease. Dwindling employer budgets. Recruitment and retention challenges. And the limitations of lifestyle programs originally developed to succeed in-person rather than online. Katie Palmer reports on how the program adapted.
Read more here
medical devices
FDA approves home spinal cord stimulator
Rose Broderick writes: The Food and Drug Administration green-lit home use of a device that helps people with spinal cord injuries regain mobility. Onward Medical announced Monday that the company had received clearance to expand the use of its spinal cord stimulator outside of clinics.
More than 60 clinics have purchased the device, according to Onward, but home use could dramatically expand the pool of potential users — more than 300,000 people in the United States live with a spinal cord injury. First cleared by the FDA for clinical use in 2024, the device delivers small zaps along the spine. In one trial, it boosted hand and arm function by 72% of participants when paired with rehabilitative therapy.
The news is the latest sign that physical rehab is not the only option for people with these disabilities — electrical stimulation can also provide relief and restore function.
Read more here
Analysis probes smartwatch accuracy
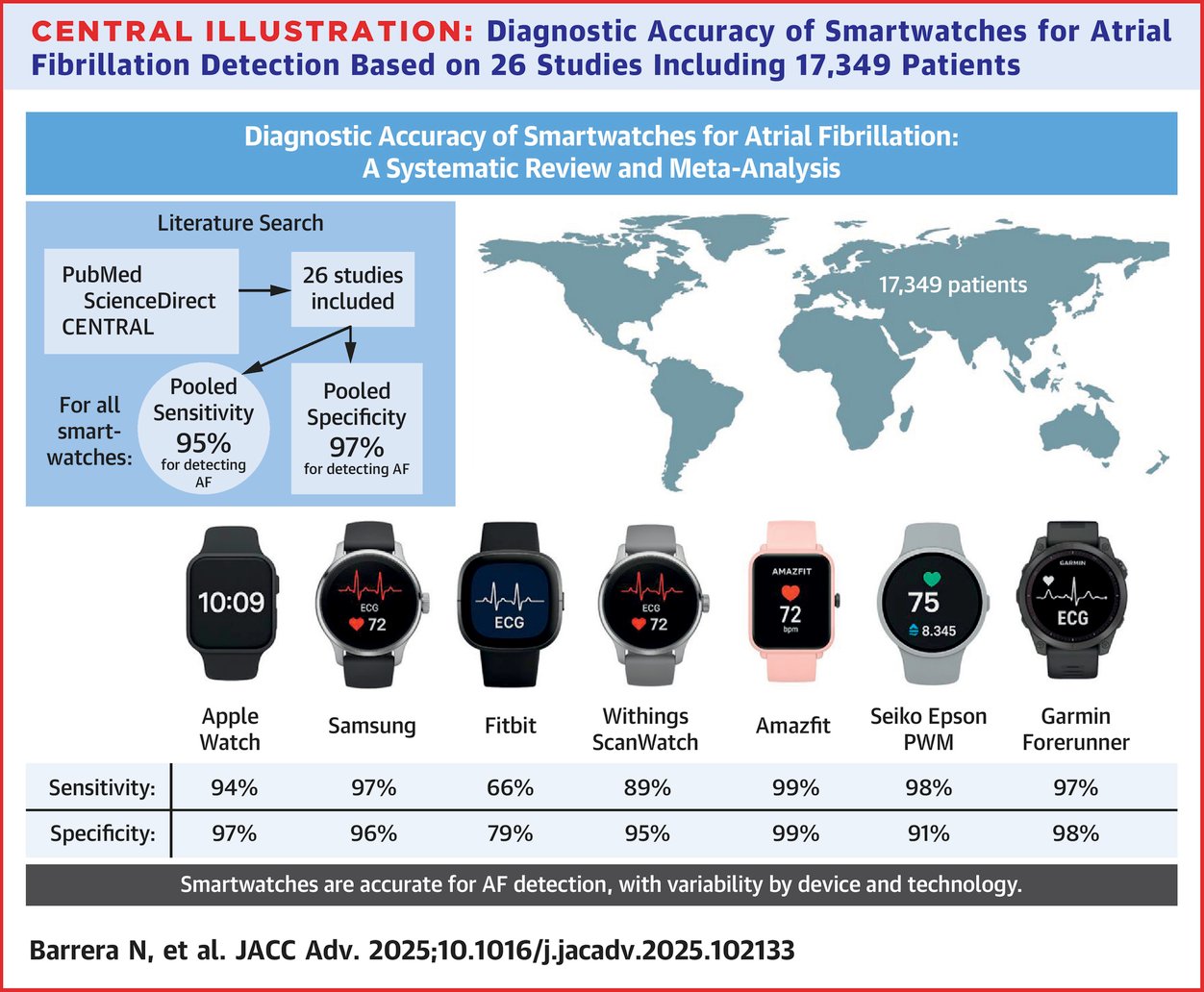
Seven years after Apple released a smartwatch feature that can detect possible atrial fibrillation in users, similar technology has become commonplace on a range of wearables. A new meta-analysis of 26 studies of devices that detect the arrhythmia concludes that the watches "possess excellent diagnostic accuracy."
Indeed, in the figure above you can see that most of the devices included in the review correctly identify people who do and don't have A-fib a high percentage of the time. An ongoing concern about detection algorithms built into widely used consumer devices is the false positive rate — or people incorrectly told they may have a condition. Even a low rate magnified over millions of devices could mean headaches for your local cardiologist.
Recently I reported on the data behind Apple's new feature that detects possible hypertension in users. It misses roughly half of cases and even then experts believe it will have a positive impact by nudging more people into treatment.


No comments