Mental health
What to know about the FDA's therapy bots meeting
The Food and Drug Administration is considering whether and how to regulate therapy chatbots that are based on large language models. Today, the agency's Digital Health Advisory Committee is meeting to consider the topic. In a new story, I explain what's going on, including some fresh insider intel.
The FDA wants to provide more clarity to developers of generative AI medical devices about what needs regulatory green light and how to get it. The agency is also also worried about LLM-based therapy bots that can provide unpredictable outputs. Regulators are aware about the growing concerns around general purpose bots like ChatGPT, which have been linked to delusions and allegedly to suicides.
In my story, I unpack how this week's advisory committee meeting might actually lead to the clarity developers have been seeking — even under a Trump administration not inclined to make new rules. There are a number of proposals on the table.
Read more here
medical devices
Brain implant developer Synchron raises $200 million
Rose Broderick writes: Synchron this morning anounced a $200 million Series D funding round to fund a U.S. clinical trial for its brain-computer interface device that helps people with paralysis navigate digital devices like smartphones. The fresh cash infusion will also help the New York-based company develop devices that can reach beyond the motor cortex area of the brain, known as a whole-brain BCI system.
Synchron is one of a handful of companies within spitting distance of FDA approval after demonstrating that their devices are safe for users. The company has zigged while their peers have zagged to capture brain activity, however, pinning their hopes on an endovascular device that snakes into the brain via a catheter. Companies like Elon Musk's Neuralink implant electrodes by making a small opening in the skull to access the brain.
Investor interest in Synchron is a sign of growing momentum in a BCI field that was considered an academic thought experiment a decade ago. Now, Wall Street analysts say there could be a $400 billion market for BCI devices, which could potentially help people with amyotrophic lateral sclerosis or other disabilities regain some autonomy. Neuralink recently announced its own $650 million Series E.
BCI's still face some significant obstacles before it can reach patients. Industry hasn't honed in on which clinical outcome assessments are most relevant for FDA regulators who evaluate whether devices like Synchron's Stentrode actually help disabled people. (Rose will have more on this topic soon. Feel free to reach out with tips or ideas: rose.broderick@statnews.com.)
research
RPM provides significant boost in practice revenues
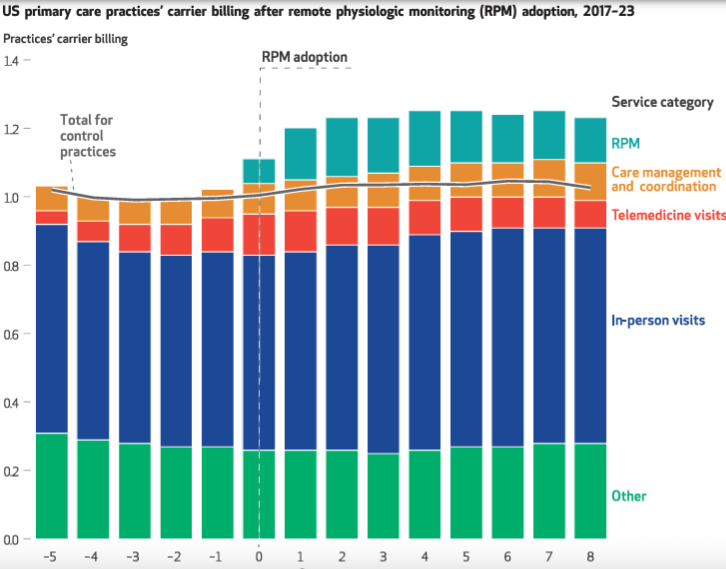
Thanks to reimbursement from Medicare and commercial payers, a growing number of primary care practices are remotely monitoring patient conditions like hypertension, using blood pressure cuffs and other connected devices. A new study in Health Affairs found that practices that adopted the technology saw an an average 20% increase in Medicare revenues compared to similar control practices. The researchers suggest a corresponding increase across all Medicare beneficiaries would lead to $6 billion in new spending. Another interesting finding: Increasing RPM use did not cause practice panels to shrink — in fact adopting practices saw more patients.
policy Medicare finalizes new tech payment policies
Medicare regulators last week finalized 2026 physician payment policies that were proposed back in July. I wrote about them in STAT Health Tech back then, in case you want to go review some of the details. In short:
- CMS finalized payment for FDA-cleared digital therapeutics for attention deficit hyperactivity disorder using the new framework launched earlier this year for digital mental health devices.
- CMS finalized an expansion of payment for remote monitoring services for situations where they collect less data and spend less time interacting with patients. I wrote previously about how this expansion is happening despite the growing concern that there ought to be additional safeguards to prevent excessive RPM billing.


No comments